Copper Ii Sulfate Sodium Hydroxide Word Equation . two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The result is a blue precipitate. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4.
from www.numerade.com
The result is a blue precipitate. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water.
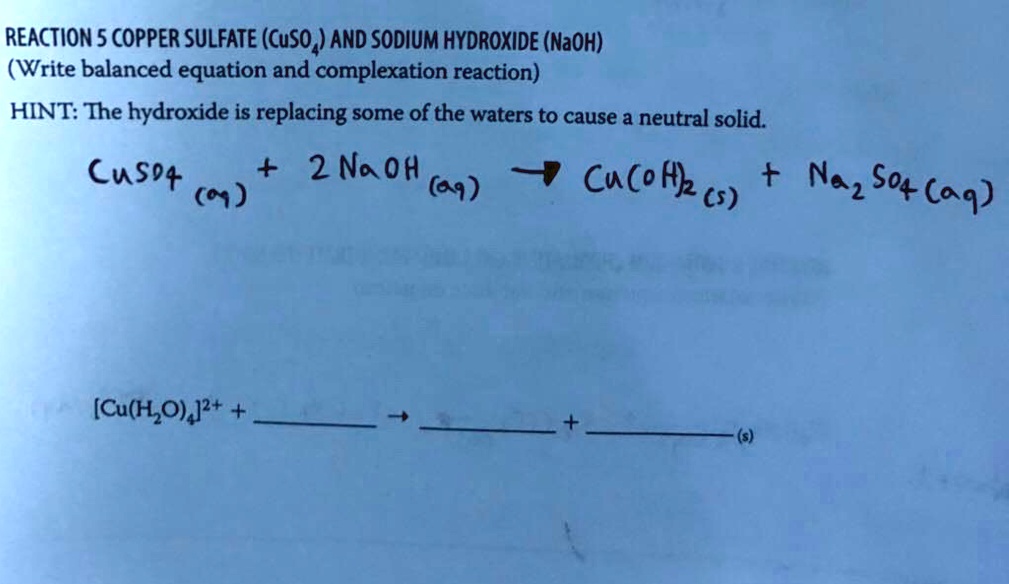
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write
Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). The result is a blue precipitate. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water.
From www.numerade.com
SOLVED Write balanced equations for each of the following reactions Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. here, copper. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From askfilo.com
1. Copper (II) sulphate solution reacts with sodium hydroxide solution to.. Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: here, copper (ii) sulfate (cuso 4) is added to. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper Ii Sulfate Sodium Hydroxide Word Equation i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. sodium hydroxide + copper. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.slideshare.net
Chemical Reactions Copper Ii Sulfate Sodium Hydroxide Word Equation The result is a blue precipitate. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: . Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). The result is a blue precipitate. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: i tried reacting copper sulfate with. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From exyamycwi.blob.core.windows.net
Iron And Copper Ii Chloride Net Ionic Equation at Mary Echols blog Copper Ii Sulfate Sodium Hydroxide Word Equation here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. The result is a blue precipitate. . Copper Ii Sulfate Sodium Hydroxide Word Equation.
From cartoondealer.com
Sodium Hydroxide Pellets, Zinc Granules And Copper(II) Sulfate In Test Copper Ii Sulfate Sodium Hydroxide Word Equation i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. enter a chemical equation and press the balance button to get the balanced equation and the type of. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.numerade.com
SOLVED 4) Copper (II) Sulfate Sodium Carbonate Complete Molecular Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. The result is a blue precipitate. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. sodium hydroxide +. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.youtube.com
How to Write the Net Ionic Equation for K2S + Cu(NO3)2 = KNO3 + CuS Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide + copper (ii) sulfate → balanced chemical. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.slideshare.net
Acids And Bases Copper Ii Sulfate Sodium Hydroxide Word Equation The result is a blue precipitate. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. . Copper Ii Sulfate Sodium Hydroxide Word Equation.
From stock.adobe.com
Double displacement reaction sodium hydroxide and copper sulfate Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. The result is a blue precipitate. in this video we'll balance the equation cuso4 + naoh = cu. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. enter a chemical equation and press the balance button to get the. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved in an aqueous solution of sodium. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Copper Ii Sulfate Sodium Hydroxide Word Equation The result is a blue precipitate. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous.. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a Copper Ii Sulfate Sodium Hydroxide Word Equation sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. enter a chemical equation and press the balance button to. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From fyohytssx.blob.core.windows.net
Sodium Hydroxide In Liquid Form at John Lucas blog Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. sodium hydroxide + copper. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From hxelaptcw.blob.core.windows.net
Copper Ii Sulfate Sodium Phosphate Balanced Equation at Raymond Horan blog Copper Ii Sulfate Sodium Hydroxide Word Equation sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: The result is a blue precipitate. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper(ii). Copper Ii Sulfate Sodium Hydroxide Word Equation.
From giolompon.blob.core.windows.net
Iron Iii Chloride Sodium Hydroxide Net Ionic Equation at Fran Swain blog Copper Ii Sulfate Sodium Hydroxide Word Equation sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper(ii) sulfate pentahydrate + sodium hydroxide =. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From cartoondealer.com
Copper Sulfate Formula On Waterdrop Background Stock Photography Copper Ii Sulfate Sodium Hydroxide Word Equation two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. here, copper (ii) sulfate (cuso. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From brainly.in
What are the products of Copper sulphate + sodium hydroxide? Brainly.in Copper Ii Sulfate Sodium Hydroxide Word Equation i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. enter a chemical equation and press the balance button to get the balanced equation and the type of. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.alamy.com
Copper(II) sulfate and Sodium Hydroxide Pellets in test tube with plug Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. enter a chemical equation. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.chegg.com
Solved Copper(II) sulfate + Sodium hydroxide Blue gelatinous Copper Ii Sulfate Sodium Hydroxide Word Equation i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The result is a blue precipitate. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. in this video we'll balance the equation cuso4 + naoh = cu (oh)2. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Sulfate Sodium Hydroxide Word Equation i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. in this video we'll. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.toppr.com
TUILU Idulal WILL SULAULLE 3 Write the chemical formula of the Copper Ii Sulfate Sodium Hydroxide Word Equation The result is a blue precipitate. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. two moles of aqueous sodium hydroxide plus one mole of aqueous copper. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Copper Ii Sulfate Sodium Hydroxide Word Equation here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). The result is a blue precipitate. Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. when carbon dioxide is dissolved. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.chegg.com
Solved copper (II) sulfate + sodium hydroxide → Blue Copper Ii Sulfate Sodium Hydroxide Word Equation two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. The result is a. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From fyocftwbd.blob.core.windows.net
Copper Chloride With Sodium Hydroxide at Walker Francisco blog Copper Ii Sulfate Sodium Hydroxide Word Equation Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The result is a blue precipitate. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. enter a chemical. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Copper Ii Sulfate Sodium Hydroxide Word Equation The result is a blue precipitate. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. enter a chemical equation and press the balance button to get the balanced equation and the type. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From hxelaptcw.blob.core.windows.net
Copper Ii Sulfate Sodium Phosphate Balanced Equation at Raymond Horan blog Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The result is a blue precipitate. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). . Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.numerade.com
SOLVED If possible, write a balanced net ionic equation for copper(II Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From cristianmeowterry.blogspot.com
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate Copper Ii Sulfate Sodium Hydroxide Word Equation sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. enter a chemical equation and press the balance button to get the. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From fyocftwbd.blob.core.windows.net
Copper Chloride With Sodium Hydroxide at Walker Francisco blog Copper Ii Sulfate Sodium Hydroxide Word Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). The result is a blue precipitate. . Copper Ii Sulfate Sodium Hydroxide Word Equation.
From nghenhansu.edu.vn
Top 94+ Images Sodium Hydroxide And Copper(ii) Nitrate Sharp Copper Ii Sulfate Sodium Hydroxide Word Equation i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper(ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. The result is a blue precipitate. when carbon dioxide is dissolved. Copper Ii Sulfate Sodium Hydroxide Word Equation.
From www.slideserve.com
PPT Evidence of Chemical Change Laboratory PowerPoint Presentation Copper Ii Sulfate Sodium Hydroxide Word Equation here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation spectator ions: two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. when carbon dioxide is dissolved in. Copper Ii Sulfate Sodium Hydroxide Word Equation.