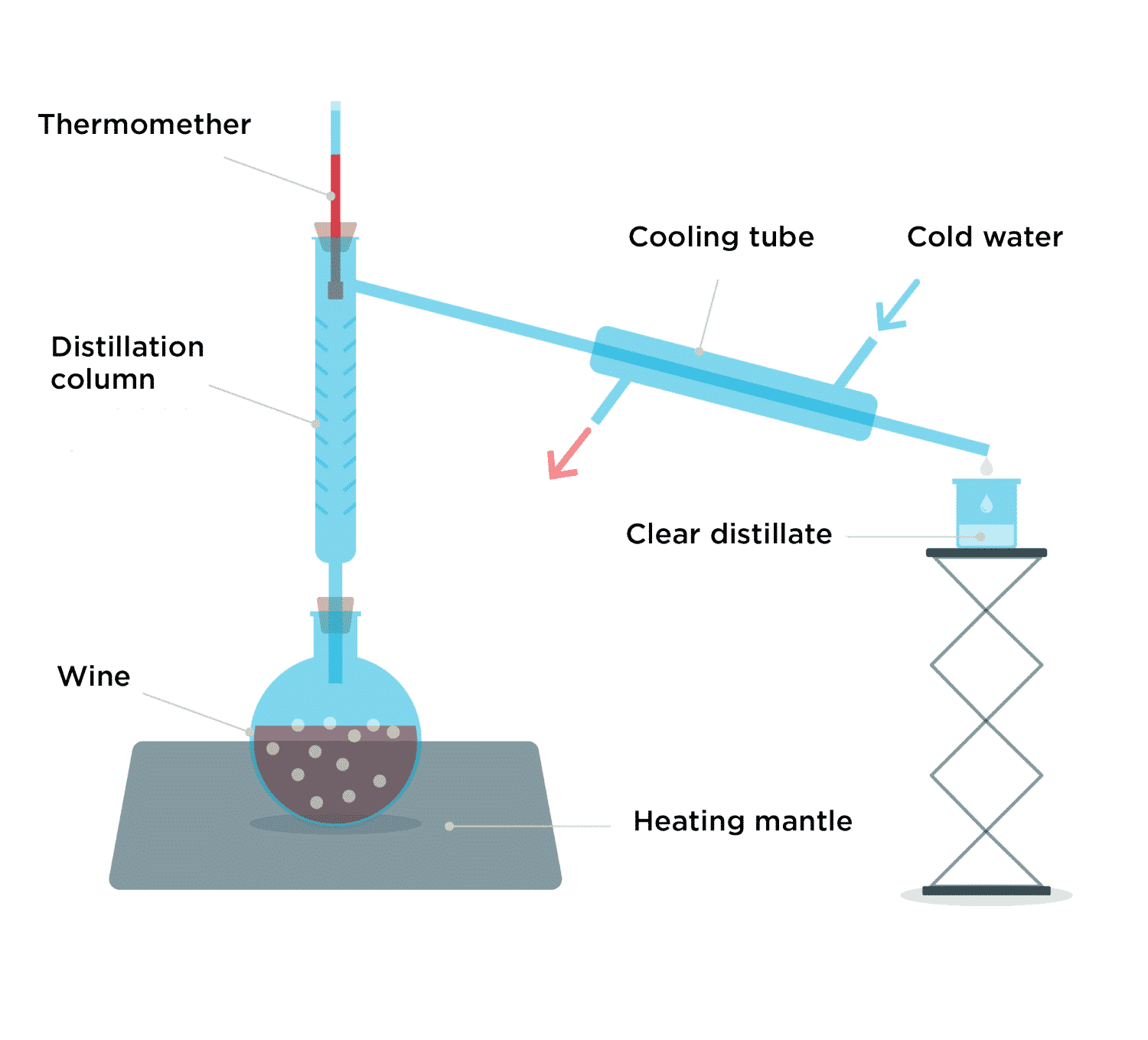
Distillation Ex . simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. There are different ways to separate mixtures, for example by. We utilize the difference in boiling points. distillation is the process of separating components of a mixture based on different boiling. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is a purification technique for a liquid or a mixture of liquids. Distillation involves boiling the solution and. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. 705 shows the behaviour of three different liquid mixtures during a simple distillation.
from mavink.com
distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. We utilize the difference in boiling points. 705 shows the behaviour of three different liquid mixtures during a simple distillation. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is a purification technique for a liquid or a mixture of liquids. There are different ways to separate mixtures, for example by. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. Distillation involves boiling the solution and. distillation is the process of separating components of a mixture based on different boiling.
Distillation Phase Diagram
Distillation Ex There are different ways to separate mixtures, for example by. There are different ways to separate mixtures, for example by. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. Distillation involves boiling the solution and. We utilize the difference in boiling points. 705 shows the behaviour of three different liquid mixtures during a simple distillation. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is a purification technique for a liquid or a mixture of liquids. distillation is the process of separating components of a mixture based on different boiling. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of.
From chemistnotes.com
Distillation Definition and Types of Distillation Chemistry Notes Distillation Ex distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is a purification technique for a liquid or a mixture of liquids. There are different ways to separate mixtures, for example by. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart.. Distillation Ex.
From mavink.com
Distillation Separating Mixtures Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is the process of separating components of a mixture based on different boiling. simple distillation is used to separate liquids whose. Distillation Ex.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. There are different ways to separate mixtures, for example by. distillation is the process of separating components of a mixture based on different boiling. . Distillation Ex.
From www.geeksforgeeks.org
Distillation Definition, Meaning, Principle, Types & Uses Distillation Ex There are different ways to separate mixtures, for example by. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. We utilize the difference in boiling points. distillation is a purification technique for a liquid or a mixture of liquids. Overview of distillation several distillation variations are used in the. Distillation Ex.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and. We utilize the difference in boiling points. distillation, the process involving the conversion of a liquid. Distillation Ex.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. We utilize the difference in boiling points.. Distillation Ex.
From mavink.com
Distillation Labeled Diagram Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. 705 shows the behaviour of three different liquid mixtures during a simple distillation. distillation is a purification technique for a liquid or a mixture of liquids. distillation is a separation technique used to separate liquid (the solvent) from a mixture and. Distillation Ex.
From aimhigh.space
Distillation and Chromatography AimHigh! Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. Distillation involves boiling the solution and. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. 705 shows the behaviour of three different liquid mixtures during a simple distillation. There are different ways to. Distillation Ex.
From www.shutterstock.com
diagramme de distillation simple en chimie image vectorielle de stock Distillation Ex Distillation involves boiling the solution and. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. 705 shows the behaviour of three different liquid mixtures during a simple distillation. There are different ways to separate mixtures, for example by. distillation is the process of separating components of a mixture based on different. Distillation Ex.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Distillation Ex simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. Distillation involves boiling the solution and. We utilize the difference in boiling points. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is the process of separating components of a. Distillation Ex.
From testbook.com
team Distillation Principle, Working, Advantages & Application Distillation Ex distillation is the process of separating components of a mixture based on different boiling. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. There are different ways to separate mixtures, for example by. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to. Distillation Ex.
From mavink.com
Distillation Phase Diagram Distillation Ex 705 shows the behaviour of three different liquid mixtures during a simple distillation. We utilize the difference in boiling points. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. Distillation involves boiling the. Distillation Ex.
From www.futura-sciences.com
Définition Distillation Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. Distillation involves boiling the solution and. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the. Distillation Ex.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Ex distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. distillation is the process of separating components of a mixture based on different boiling. 705 shows. Distillation Ex.
From byjus.com
Distillation Definition, Types & Applications Chemistry BYJU'S Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation is a purification technique for a liquid or a mixture of liquids. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation, the process involving the conversion of a. Distillation Ex.
From mavink.com
Diagram Of Distillation Distillation Ex simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. We utilize the difference in boiling points. There are different ways to separate mixtures, for example by. distillation is a purification technique for a liquid or a mixture of liquids. distillation is the process of separating components of a mixture based. Distillation Ex.
From ar.inspiredpencil.com
Distillation Diagram Distillation Ex We utilize the difference in boiling points. Distillation involves boiling the solution and. distillation is a purification technique for a liquid or a mixture of liquids. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. There are different ways to separate mixtures, for example by. distillation is a separation technique. Distillation Ex.
From ar.inspiredpencil.com
Distillation Process Distillation Ex distillation is the process of separating components of a mixture based on different boiling. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. We utilize the difference in boiling points. distillation is a purification technique for a liquid or a mixture of liquids. There are different ways to. Distillation Ex.
From pharmaguides.in
1.5 What Is Distillation ? Read Everything About It Distillation Ex There are different ways to separate mixtures, for example by. Distillation involves boiling the solution and. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points. simple distillation is. Distillation Ex.
From www.geeksforgeeks.org
Separation by Distillation Distillation Ex distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is the process of separating components of a mixture based on different boiling. There are different ways to separate mixtures, for example by. simple distillation is used to separate liquids whose boiling points are at least 100. Distillation Ex.
From www.chemicalslearning.com
Simple Distillation Process Differential Distillation Process Distillation Ex simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. distillation is a purification technique for a liquid or a mixture of liquids. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. 705 shows the behaviour of three different liquid mixtures during. Distillation Ex.
From www.lelivrescolaire.fr
Distillation. Distillation Ex distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is the process of separating components of a mixture based on different boiling. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. distillation is a purification technique for a liquid. Distillation Ex.
From physique-chimie-college.fr
La distillation Distillation Ex 705 shows the behaviour of three different liquid mixtures during a simple distillation. distillation is the process of separating components of a mixture based on different boiling. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. simple distillation is used to separate liquids whose boiling points are at least 100. Distillation Ex.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Distillation Ex We utilize the difference in boiling points. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. There are different ways to separate mixtures, for example by. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. Overview of distillation several distillation variations. Distillation Ex.
From issr.edu.kh
Distillation Distillation Ex distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points. Distillation involves boiling the solution and. There are different ways to separate mixtures, for example by. distillation, the. Distillation Ex.
From en.wikipedia.org
Distillation Wikipedia Distillation Ex Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is a purification technique for a liquid or a mixture of liquids. Distillation involves boiling the solution and. distillation, the. Distillation Ex.
From www.aquaportail.com
Distillation définition et explications Distillation Ex Distillation involves boiling the solution and. There are different ways to separate mixtures, for example by. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is the process. Distillation Ex.
From www.dreamstime.com
Simple Distillation Apparatus Diagram with Full Process Vector Distillation Ex There are different ways to separate mixtures, for example by. distillation is the process of separating components of a mixture based on different boiling. Distillation involves boiling the solution and. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is a purification technique for a liquid. Distillation Ex.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Distillation Ex We utilize the difference in boiling points. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. 705 shows the behaviour of three different liquid mixtures during a simple distillation. Distillation involves boiling the solution and. distillation is a purification technique for a liquid or a mixture of liquids. There are different. Distillation Ex.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Distillation Ex There are different ways to separate mixtures, for example by. We utilize the difference in boiling points. Distillation involves boiling the solution and. distillation is a purification technique for a liquid or a mixture of liquids. 705 shows the behaviour of three different liquid mixtures during a simple distillation. distillation, the process involving the conversion of a liquid. Distillation Ex.
From jupiter.plymouth.edu
DISTILLATION APPARATUS Distillation Ex Distillation involves boiling the solution and. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. 705 shows the behaviour of three different liquid mixtures during a simple distillation. We utilize the difference in boiling points. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep. Distillation Ex.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds Distillation Ex Distillation involves boiling the solution and. We utilize the difference in boiling points. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. There are different ways to separate mixtures, for example by. distillation is the process of separating components of a mixture based on different boiling. distillation, the process involving. Distillation Ex.
From www.thoughtco.com
What Is Distillation? Principles and Uses Distillation Ex distillation is a purification technique for a liquid or a mixture of liquids. simple distillation is used to separate liquids whose boiling points are at least 100 degrees apart. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. 705 shows the behaviour of three different liquid mixtures during a simple. Distillation Ex.
From www.researchgate.net
Schéma explicatif de l'étape de distillation Download Scientific Diagram Distillation Ex We utilize the difference in boiling points. There are different ways to separate mixtures, for example by. 705 shows the behaviour of three different liquid mixtures during a simple distillation. distillation is a purification technique for a liquid or a mixture of liquids. distillation is the process of separating components of a mixture based on different boiling. . Distillation Ex.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记3.5.2 Reactions of Alcohols翰林国际教育 Distillation Ex distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Overview of distillation several distillation variations are used in the organic laboratory depending on the properties of. distillation is the. Distillation Ex.