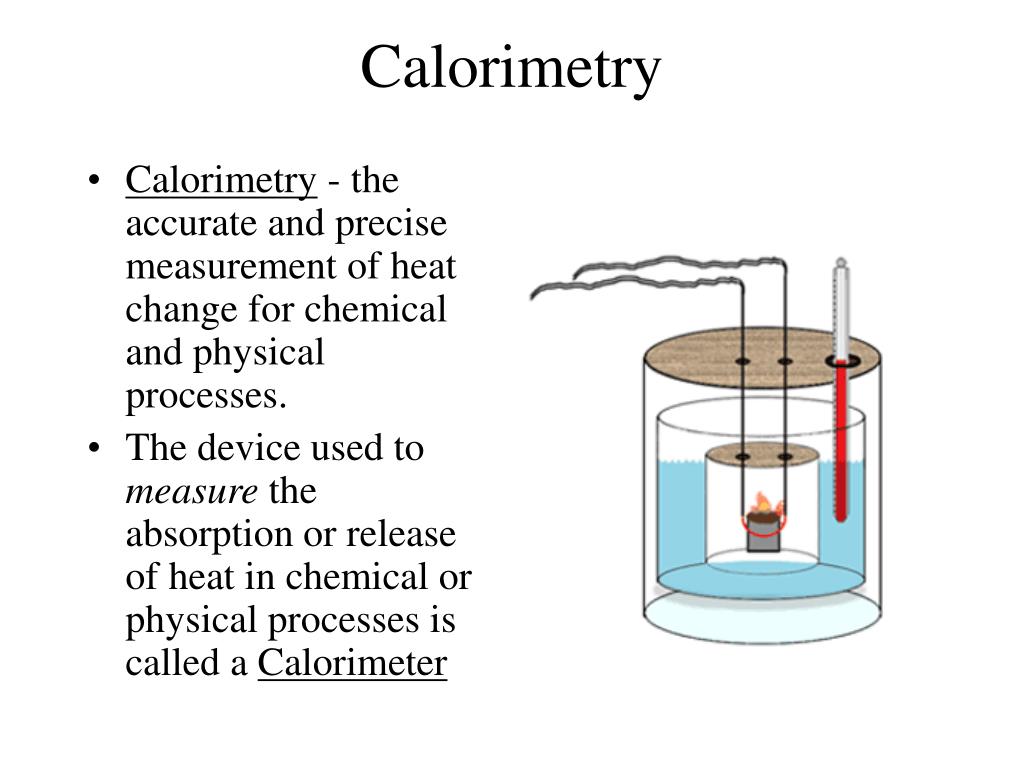
Calorimetry Definition Chemistry . Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explore different types of calorimeters, endothermic. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic.
from www.slideserve.com
For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. By knowing the change in. Explore different types of calorimeters, endothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. For example, when an exothermic.
PPT Chapter 11 PowerPoint Presentation, free download ID1084959
Calorimetry Definition Chemistry By knowing the change in. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Explore different types of calorimeters, endothermic. For example, when an exothermic. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. For example, when an exothermic.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Definition Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Explore different types of calorimeters, endothermic. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is. Calorimetry Definition Chemistry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Definition Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry Definition Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Definition Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. Learn how to measure the amount of heat involved in a physical. Calorimetry Definition Chemistry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Definition Chemistry By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during. Calorimetry Definition Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Definition Chemistry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Explore different types of calorimeters, endothermic. Calorimetry describes a set of techniques employed to. Calorimetry Definition Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Definition Chemistry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry describes a set. Calorimetry Definition Chemistry.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Definition Chemistry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Explore different types of calorimeters, endothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. Calorimetry Definition Chemistry.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Definition Chemistry By knowing the change in. Explore different types of calorimeters, endothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the amount. Calorimetry Definition Chemistry.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetry Definition Chemistry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation.. Calorimetry Definition Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Definition Chemistry For example, when an exothermic. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. By knowing the change in. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is a device used to measure the amount of heat. Calorimetry Definition Chemistry.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimetry Definition Chemistry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Explore different types of calorimeters, endothermic. Learn how to measure the amount of heat involved in a physical or. Calorimetry Definition Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Definition Chemistry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. By knowing the change in. A calorimeter is a device used to measure the. Calorimetry Definition Chemistry.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimetry Definition Chemistry By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters. Calorimetry Definition Chemistry.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimetry Definition Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during. Calorimetry Definition Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Definition Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Explore different types of calorimeters, endothermic. For example, when an exothermic. Learn how to measure the amount of heat. Calorimetry Definition Chemistry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry Definition Chemistry Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is. Calorimetry Definition Chemistry.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Definition Chemistry Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry Definition Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Definition Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called.. Calorimetry Definition Chemistry.
From users.highland.edu
Calorimetry Calorimetry Definition Chemistry Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of. Calorimetry Definition Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Definition Chemistry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a. Calorimetry Definition Chemistry.
From guweb2.gonzaga.edu
CHEM 101 General Chemistry topic Calorimetry Definition Chemistry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Explore different types of calorimeters, endothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Learn how to measure the amount of heat. Calorimetry Definition Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Definition Chemistry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Calorimetry Definition Chemistry.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Definition Chemistry For example, when an exothermic. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of. Calorimetry Definition Chemistry.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Definition Chemistry For example, when an exothermic. Explore different types of calorimeters, endothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. By knowing the change in. A calorimeter is a device used to measure the amount. Calorimetry Definition Chemistry.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Definition Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. Learn how to measure the amount of. Calorimetry Definition Chemistry.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Definition Chemistry For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. By knowing the change. Calorimetry Definition Chemistry.
From studylib.net
Calorimetry Calorimetry Definition Chemistry For example, when an exothermic. Explore different types of calorimeters, endothermic. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is a device used. Calorimetry Definition Chemistry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Definition Chemistry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. By knowing the change in. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of. Calorimetry Definition Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Definition Chemistry For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explore different types of calorimeters, endothermic. For example, when an exothermic. Calorimetry describes a. Calorimetry Definition Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Definition Chemistry For example, when an exothermic. By knowing the change in. For example, when an exothermic. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Explore different types of calorimeters, endothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A. Calorimetry Definition Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Definition Chemistry Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. For example, when an exothermic. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a. Calorimetry Definition Chemistry.
From quizunderslung.z4.web.core.windows.net
What Is Direct Calorimetry Calorimetry Definition Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explore different types of calorimeters, endothermic. For example, when an exothermic. For example, when. Calorimetry Definition Chemistry.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Calorimetry Definition Chemistry Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. For example, when an exothermic. Explore different types of calorimeters, endothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount. Calorimetry Definition Chemistry.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Definition Chemistry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set of techniques employed to measure enthalpy changes in. Calorimetry Definition Chemistry.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Definition Chemistry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or. Calorimetry Definition Chemistry.