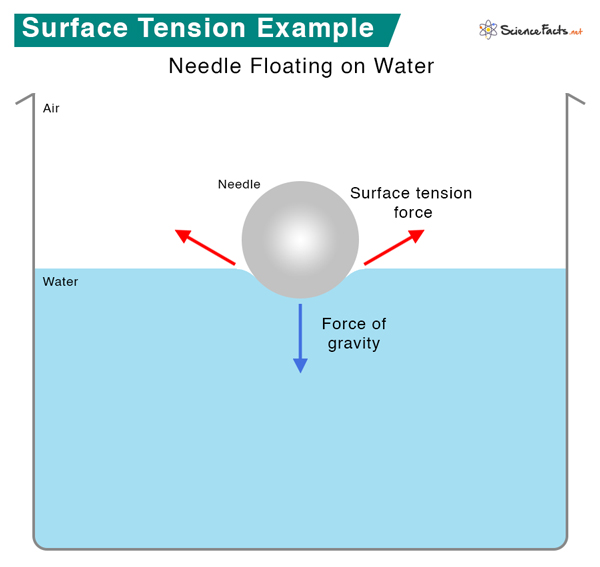
Does Surface Tension Increase With Viscosity . Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Compared to viscosity, surface tension is a simpler phenomenon. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? The answer lies in a property called surface tension, which depends on intermolecular forces. Liquids with strong intermolecular forces have higher. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. This is caused by the friction between molecules. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. It is basically stable, changed mostly by temperature and chemicals.
from www.sciencefacts.net
Compared to viscosity, surface tension is a simpler phenomenon. This is caused by the friction between molecules. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. It is basically stable, changed mostly by temperature and chemicals. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. The answer lies in a property called surface tension, which depends on intermolecular forces. Liquids with strong intermolecular forces have higher. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? The surface tension of a liquid is a measure of the elastic force in the liquid's surface.
Surface Tension Definition, Examples, and Unit
Does Surface Tension Increase With Viscosity Compared to viscosity, surface tension is a simpler phenomenon. Liquids with strong intermolecular forces have higher. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Compared to viscosity, surface tension is a simpler phenomenon. The answer lies in a property called surface tension, which depends on intermolecular forces. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. It is basically stable, changed mostly by temperature and chemicals. This is caused by the friction between molecules. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film?
From lessonlibrarybuglers.z21.web.core.windows.net
Viscosity Vs Surface Tension Does Surface Tension Increase With Viscosity The surface tension of a liquid is a measure of the elastic force in the liquid's surface. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Liquids with strong intermolecular forces have higher. This is caused by the. Does Surface Tension Increase With Viscosity.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Does Surface Tension Increase With Viscosity Liquids with strong intermolecular forces have higher. This is caused by the friction between molecules. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car. Does Surface Tension Increase With Viscosity.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Does Surface Tension Increase With Viscosity The answer lies in a property called surface tension, which depends on intermolecular forces. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. It is basically stable, changed mostly by temperature and chemicals. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a. Does Surface Tension Increase With Viscosity.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Does Surface Tension Increase With Viscosity Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. The answer lies in a property called surface tension, which depends on intermolecular forces. This is caused by the friction between molecules. The surface tension of a liquid is a measure of the elastic force in the. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
Surface tension and viscosity of various sample solutions Download Table Does Surface Tension Increase With Viscosity It is basically stable, changed mostly by temperature and chemicals. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
Dependence of polymer solution viscosity (μ) and surface tension (γ) on Does Surface Tension Increase With Viscosity The answer lies in a property called surface tension, which depends on intermolecular forces. Compared to viscosity, surface tension is a simpler phenomenon. This is caused by the friction between molecules. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. It is basically stable, changed mostly. Does Surface Tension Increase With Viscosity.
From www.diplomageeks.com
Define capilirity, viscosity, coefficient of viscosity and surface Does Surface Tension Increase With Viscosity This is caused by the friction between molecules. Liquids with strong intermolecular forces have higher. The answer lies in a property called surface tension, which depends on intermolecular forces. It is basically stable, changed mostly by temperature and chemicals. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
(a) Viscosity, (b) surface tension and (c) Z number of the solvent Does Surface Tension Increase With Viscosity It is basically stable, changed mostly by temperature and chemicals. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. The answer lies in a property called surface tension, which depends. Does Surface Tension Increase With Viscosity.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Does Surface Tension Increase With Viscosity Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Compared to viscosity, surface tension is a simpler phenomenon. It is basically stable, changed mostly by temperature and chemicals. The answer lies in a property called surface tension, which depends on intermolecular forces. The surface tension of. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
Surface tension of solution, vapor pressure of water, viscosity of Does Surface Tension Increase With Viscosity It is basically stable, changed mostly by temperature and chemicals. This is caused by the friction between molecules. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. The answer lies in a property called surface tension, which depends on intermolecular forces. If liquids tend to adopt. Does Surface Tension Increase With Viscosity.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Does Surface Tension Increase With Viscosity Liquids with strong intermolecular forces have higher. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Having a low surface tension. Does Surface Tension Increase With Viscosity.
From www.slideserve.com
PPT Biomedical importance of surface tension & Viscosity PowerPoint Does Surface Tension Increase With Viscosity Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Compared to viscosity, surface tension is a simpler phenomenon. Liquids with strong intermolecular forces have higher. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing. Does Surface Tension Increase With Viscosity.
From sophie-ksullivan.blogspot.com
Surface Tension and Viscosity Are Used to Describe Does Surface Tension Increase With Viscosity Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Liquids with strong intermolecular forces have higher. This is caused by the friction between molecules. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the.. Does Surface Tension Increase With Viscosity.
From www.etutorworld.com
Viscosity and Surface Tension eTutorWorld Does Surface Tension Increase With Viscosity The surface tension of a liquid is a measure of the elastic force in the liquid's surface. This is caused by the friction between molecules. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. It is basically stable, changed mostly by temperature and chemicals. Compared to. Does Surface Tension Increase With Viscosity.
From www.vrogue.co
A Variation Of The Surface Tension And Viscosity Of T vrogue.co Does Surface Tension Increase With Viscosity The surface tension of a liquid is a measure of the elastic force in the liquid's surface. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Liquids with strong intermolecular forces have higher. Firstly, surface tension is related. Does Surface Tension Increase With Viscosity.
From www.slideserve.com
PPT Biomedical importance of surface tension & Viscosity PowerPoint Does Surface Tension Increase With Viscosity This is caused by the friction between molecules. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
The density, surface tension, and kinematic viscosity of waterfuel at Does Surface Tension Increase With Viscosity If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Having a low surface tension when the alveoli are small prevents surface. Does Surface Tension Increase With Viscosity.
From www.youtube.com
Chemistry Explained Viscosity, Surface Tension, Adhesion, Cohesion Does Surface Tension Increase With Viscosity Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Liquids with strong intermolecular forces have higher. The answer lies in a property called surface tension, which depends on intermolecular forces. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
Variations of (a) the viscosity, (b) surface tension and (c) density Does Surface Tension Increase With Viscosity Compared to viscosity, surface tension is a simpler phenomenon. Liquids with strong intermolecular forces have higher. The answer lies in a property called surface tension, which depends on intermolecular forces. This is caused by the friction between molecules. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car. Does Surface Tension Increase With Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity 1 Studypool Does Surface Tension Increase With Viscosity Liquids with strong intermolecular forces have higher. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. It is basically stable, changed mostly by temperature. Does Surface Tension Increase With Viscosity.
From collegedunia.com
Viscosity Definition, Formula, Types & Examples Does Surface Tension Increase With Viscosity The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Liquids with strong intermolecular forces have higher. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. The answer lies in a property called surface tension, which depends on intermolecular forces.. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
Variation of the viscosity and surface tension as a function of the Does Surface Tension Increase With Viscosity If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Compared to viscosity, surface tension is a simpler phenomenon. The answer lies in a property called surface tension, which depends on intermolecular forces. Liquids with strong intermolecular forces have. Does Surface Tension Increase With Viscosity.
From sophie-ksullivan.blogspot.com
Surface Tension and Viscosity Are Used to Describe Does Surface Tension Increase With Viscosity It is basically stable, changed mostly by temperature and chemicals. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Compared to viscosity, surface tension is a simpler phenomenon. The answer lies in a property called surface tension, which depends on intermolecular forces. Having a low surface. Does Surface Tension Increase With Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity 2 Studypool Does Surface Tension Increase With Viscosity Liquids with strong intermolecular forces have higher. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. It is basically stable, changed mostly by temperature. Does Surface Tension Increase With Viscosity.
From irajlorenzeno.blob.core.windows.net
Does Surface Tension Increase With Intermolecular Forces at Does Surface Tension Increase With Viscosity Compared to viscosity, surface tension is a simpler phenomenon. Liquids with strong intermolecular forces have higher. The answer lies in a property called surface tension, which depends on intermolecular forces. It is basically stable, changed mostly by temperature and chemicals. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension. Does Surface Tension Increase With Viscosity.
From www.studypool.com
SOLUTION Surface Tension & viscosity Physics Notes Studypool Does Surface Tension Increase With Viscosity This is caused by the friction between molecules. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Compared to viscosity, surface. Does Surface Tension Increase With Viscosity.
From learningschooltrkesp5v.z22.web.core.windows.net
Viscosity Vs Surface Tension Does Surface Tension Increase With Viscosity Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? The answer lies in a property. Does Surface Tension Increase With Viscosity.
From www.youtube.com
CHEM 1412 Lecture 1/131/14 Part 4 Surface Tension and Viscosity YouTube Does Surface Tension Increase With Viscosity Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. This is caused by the friction between molecules. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. If liquids tend to adopt the shapes. Does Surface Tension Increase With Viscosity.
From printablelibigergz.z21.web.core.windows.net
Viscosity Vs Surface Tension Does Surface Tension Increase With Viscosity Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Liquids with strong intermolecular forces have higher. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film?. Does Surface Tension Increase With Viscosity.
From www.youtube.com
04 Ch 12 Viscosity and Surface Tension YouTube Does Surface Tension Increase With Viscosity Liquids with strong intermolecular forces have higher. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. The answer lies in a property called surface tension, which depends on intermolecular forces. It is basically stable, changed mostly by temperature and chemicals. Firstly, surface tension is related to the. Does Surface Tension Increase With Viscosity.
From www.studypool.com
SOLUTION Surface Tension & viscosity Physics Notes Studypool Does Surface Tension Increase With Viscosity Compared to viscosity, surface tension is a simpler phenomenon. Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. The answer lies in a property called surface tension, which depends on intermolecular forces. The surface tension of a liquid is a measure of the elastic force in the. Does Surface Tension Increase With Viscosity.
From www.researchgate.net
The variation of solution viscosity and surface tension as a function Does Surface Tension Increase With Viscosity Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Compared to viscosity, surface tension is a simpler phenomenon. The answer lies in a property called surface tension, which depends on intermolecular forces. This is caused by the friction between molecules. It is basically stable, changed mostly by. Does Surface Tension Increase With Viscosity.
From www.youtube.com
Surface Tension, Viscosity and Capillary Action YouTube Does Surface Tension Increase With Viscosity The answer lies in a property called surface tension, which depends on intermolecular forces. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. It is basically stable, changed mostly by temperature and chemicals. Compared to viscosity, surface tension is a simpler phenomenon. Firstly, surface tension is related to the cohesive forces at. Does Surface Tension Increase With Viscosity.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Does Surface Tension Increase With Viscosity Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. It is basically stable, changed mostly by temperature and chemicals. The surface tension of a. Does Surface Tension Increase With Viscosity.
From www.rheologylab.com
Dip Coating Viscosity, Yield Stress and Surface Tension Rheology Lab Does Surface Tension Increase With Viscosity Having a low surface tension when the alveoli are small prevents surface tension from collapsing the alveoli, and surface tension increasing as. Firstly, surface tension is related to the cohesive forces at the liquid surface, while viscosity is associated with the internal friction within the. Liquids with strong intermolecular forces have higher. The surface tension of a liquid is a. Does Surface Tension Increase With Viscosity.