Do Co And Co2 Have The Same Empirical Formula . For example, both types of. there are two types of formulas, empirical and molecular. both have the same empirical formula, yet they are different compounds with different molecular formulas. They are also the molecular formulas. The ratios hold true on the molar level as well. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. Butene is c 4 h 8 , or four times the. The empirical formula mass for. the molecular formula and empirical formula of some substances are the same. the empirical formula tells you the simplest ratio of the various atoms present in a substance. Lowest whole number ratio of the elements in a compound. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. an empirical formula tells us the relative ratios of different atoms in a compound. For example, in ethane, c 2 h. there are the empirical formulas for carbon dioxide, ammonia, and methane.
from www.chegg.com
an empirical formula tells us the relative ratios of different atoms in a compound. there are the empirical formulas for carbon dioxide, ammonia, and methane. For example, both types of. the molecular formula and empirical formula of some substances are the same. Lowest whole number ratio of the elements in a compound. both have the same empirical formula, yet they are different compounds with different molecular formulas. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. the empirical formula tells you the simplest ratio of the various atoms present in a substance. For example, in ethane, c 2 h. The ratios hold true on the molar level as well.
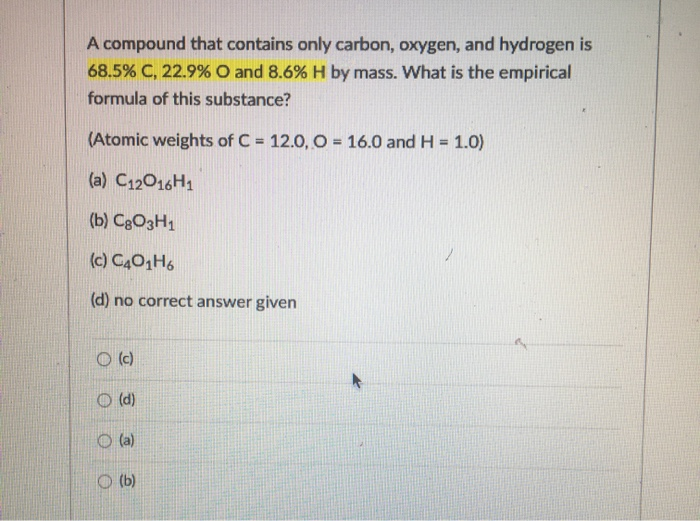
Solved A compound that contains only carbon, oxygen, and
Do Co And Co2 Have The Same Empirical Formula for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. there are the empirical formulas for carbon dioxide, ammonia, and methane. there are two types of formulas, empirical and molecular. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. They are also the molecular formulas. both have the same empirical formula, yet they are different compounds with different molecular formulas. Lowest whole number ratio of the elements in a compound. Butene is c 4 h 8 , or four times the. The ratios hold true on the molar level as well. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. an empirical formula tells us the relative ratios of different atoms in a compound. the empirical formula tells you the simplest ratio of the various atoms present in a substance. The empirical formula mass for. the molecular formula and empirical formula of some substances are the same. For example, both types of. For example, in ethane, c 2 h.
From stock.adobe.com
Carbon monoxide CO and carbon dioxide CO2 molecule models and chemical Do Co And Co2 Have The Same Empirical Formula there are the empirical formulas for carbon dioxide, ammonia, and methane. Lowest whole number ratio of the elements in a compound. the empirical formula tells you the simplest ratio of the various atoms present in a substance. They are also the molecular formulas. both have the same empirical formula, yet they are different compounds with different molecular. Do Co And Co2 Have The Same Empirical Formula.
From studylib.net
Empirical Formulas Do Co And Co2 Have The Same Empirical Formula the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. an empirical formula tells us the relative ratios of different atoms in a compound. there are two types. Do Co And Co2 Have The Same Empirical Formula.
From diagramlistopinioned.z13.web.core.windows.net
Electron Dot Formula Of Carbon Dioxide Do Co And Co2 Have The Same Empirical Formula The ratios hold true on the molar level as well. Butene is c 4 h 8 , or four times the. there are the empirical formulas for carbon dioxide, ammonia, and methane. They are also the molecular formulas. there are two types of formulas, empirical and molecular. the molecular formula and empirical formula of some substances are. Do Co And Co2 Have The Same Empirical Formula.
From www.vrogue.co
Empirical Formula Calculation From Co2 And H2o Combus vrogue.co Do Co And Co2 Have The Same Empirical Formula For example, both types of. The ratios hold true on the molar level as well. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. Lowest whole number ratio of the elements in a compound. For example, in ethane, c 2 h. for example, consider a. Do Co And Co2 Have The Same Empirical Formula.
From quizzlibrarydenna.z13.web.core.windows.net
Empirical Formulas Worksheets Do Co And Co2 Have The Same Empirical Formula both have the same empirical formula, yet they are different compounds with different molecular formulas. Butene is c 4 h 8 , or four times the. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. Lowest whole number ratio of the elements in a compound.. Do Co And Co2 Have The Same Empirical Formula.
From www.coursehero.com
[Solved] 22. Which of the following compounds have the same empirical Do Co And Co2 Have The Same Empirical Formula for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. both have the same empirical formula, yet they are different compounds with different molecular formulas. an empirical formula. Do Co And Co2 Have The Same Empirical Formula.
From www.slideserve.com
PPT Empirical Formulas PowerPoint Presentation, free download ID Do Co And Co2 Have The Same Empirical Formula the molecular formula and empirical formula of some substances are the same. They are also the molecular formulas. The empirical formula mass for. both have the same empirical formula, yet they are different compounds with different molecular formulas. For example, in ethane, c 2 h. an empirical formula tells us the relative ratios of different atoms in. Do Co And Co2 Have The Same Empirical Formula.
From scienceinfo.com
Empirical Formula Definition, Calculation Do Co And Co2 Have The Same Empirical Formula Butene is c 4 h 8 , or four times the. there are the empirical formulas for carbon dioxide, ammonia, and methane. For example, both types of. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. The ratios hold true on the molar level as. Do Co And Co2 Have The Same Empirical Formula.
From www.chegg.com
Solved Part A Which compounds do not have the same empirical Do Co And Co2 Have The Same Empirical Formula For example, in ethane, c 2 h. there are the empirical formulas for carbon dioxide, ammonia, and methane. For example, both types of. Butene is c 4 h 8 , or four times the. there are two types of formulas, empirical and molecular. the empirical formula represents the lowest whole number ratio of the elements in a. Do Co And Co2 Have The Same Empirical Formula.
From preparatorychemistry.com
Combustion Analysis Do Co And Co2 Have The Same Empirical Formula For example, in ethane, c 2 h. the empirical formula tells you the simplest ratio of the various atoms present in a substance. there are two types of formulas, empirical and molecular. The ratios hold true on the molar level as well. the empirical formula represents the lowest whole number ratio of the elements in a molecule. Do Co And Co2 Have The Same Empirical Formula.
From brainly.com
what is empirical formula ?Meaning of empirical formula Do Co And Co2 Have The Same Empirical Formula there are the empirical formulas for carbon dioxide, ammonia, and methane. They are also the molecular formulas. Butene is c 4 h 8 , or four times the. The empirical formula mass for. The ratios hold true on the molar level as well. both have the same empirical formula, yet they are different compounds with different molecular formulas.. Do Co And Co2 Have The Same Empirical Formula.
From chemifos.blogspot.com
In The Chemical Formula Of Co2. What Is The Ratio Of Carbon And Oxygen Do Co And Co2 Have The Same Empirical Formula Butene is c 4 h 8 , or four times the. The empirical formula mass for. the empirical formula tells you the simplest ratio of the various atoms present in a substance. the molecular formula and empirical formula of some substances are the same. The ratios hold true on the molar level as well. both have the. Do Co And Co2 Have The Same Empirical Formula.
From www.sliderbase.com
Empirical Formula Presentation Chemistry Do Co And Co2 Have The Same Empirical Formula Butene is c 4 h 8 , or four times the. the empirical formula tells you the simplest ratio of the various atoms present in a substance. there are two types of formulas, empirical and molecular. They are also the molecular formulas. Lowest whole number ratio of the elements in a compound. an empirical formula tells us. Do Co And Co2 Have The Same Empirical Formula.
From www.chegg.com
Solved A compound that contains only carbon, hydrogen, and Do Co And Co2 Have The Same Empirical Formula an empirical formula tells us the relative ratios of different atoms in a compound. Butene is c 4 h 8 , or four times the. there are the empirical formulas for carbon dioxide, ammonia, and methane. For example, both types of. the empirical formula represents the lowest whole number ratio of the elements in a molecule while. Do Co And Co2 Have The Same Empirical Formula.
From guidefilakvn.z14.web.core.windows.net
Dot And Cross Diagram For Carbon Dioxide Do Co And Co2 Have The Same Empirical Formula an empirical formula tells us the relative ratios of different atoms in a compound. The ratios hold true on the molar level as well. both have the same empirical formula, yet they are different compounds with different molecular formulas. there are two types of formulas, empirical and molecular. Lowest whole number ratio of the elements in a. Do Co And Co2 Have The Same Empirical Formula.
From sciencenotes.org
Empirical vs Molecular Formula Do Co And Co2 Have The Same Empirical Formula They are also the molecular formulas. both have the same empirical formula, yet they are different compounds with different molecular formulas. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. Lowest whole number ratio of the elements in a compound. the molecular formula and empirical formula of some substances are. Do Co And Co2 Have The Same Empirical Formula.
From www.youtube.com
Empirical Formulas with Combustion Analysis YouTube Do Co And Co2 Have The Same Empirical Formula For example, both types of. Butene is c 4 h 8 , or four times the. They are also the molecular formulas. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. there are two types of formulas, empirical and molecular. the empirical formula tells. Do Co And Co2 Have The Same Empirical Formula.
From www.numerade.com
SOLVED Which compounds do not have the same empirical formula? A) C2H2 Do Co And Co2 Have The Same Empirical Formula The empirical formula mass for. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. there are the empirical formulas for carbon dioxide, ammonia, and methane. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. the molecular. Do Co And Co2 Have The Same Empirical Formula.
From www.alamy.com
Carbon monoxide CO and carbon dioxide CO2 molecule. Structural chemical Do Co And Co2 Have The Same Empirical Formula an empirical formula tells us the relative ratios of different atoms in a compound. For example, in ethane, c 2 h. Butene is c 4 h 8 , or four times the. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. The ratios hold true. Do Co And Co2 Have The Same Empirical Formula.
From solvedlib.com
Determine the empirical formula for a compound that c… SolvedLib Do Co And Co2 Have The Same Empirical Formula Butene is c 4 h 8 , or four times the. both have the same empirical formula, yet they are different compounds with different molecular formulas. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. an empirical formula tells us the relative ratios of. Do Co And Co2 Have The Same Empirical Formula.
From www.chemistrystudent.com
Empirical Formula (Alevel) ChemistryStudent Do Co And Co2 Have The Same Empirical Formula for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. there are two types of formulas, empirical and molecular. an empirical formula tells us the relative ratios of different atoms in a compound. The ratios hold true on the molar level as well. For example, in ethane, c 2 h. Lowest. Do Co And Co2 Have The Same Empirical Formula.
From www.slideserve.com
PPT Empirical vs. molecular PowerPoint Presentation, free download Do Co And Co2 Have The Same Empirical Formula the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. The empirical formula mass for. The ratios hold true on the molar level as well. They are also the molecular formulas. there are the empirical formulas for carbon dioxide, ammonia, and methane. the molecular formula. Do Co And Co2 Have The Same Empirical Formula.
From learningschoolohhoyy6h.z22.web.core.windows.net
Empirical Formulas And Molecular Formulas Do Co And Co2 Have The Same Empirical Formula The ratios hold true on the molar level as well. The empirical formula mass for. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. both have the same empirical formula, yet they are different compounds with different molecular formulas. For example, in ethane, c 2 h. there are the empirical. Do Co And Co2 Have The Same Empirical Formula.
From geteducationbee.com
Empirical Formula Definition, Calculator, Best Examples Get Education Bee Do Co And Co2 Have The Same Empirical Formula The empirical formula mass for. The ratios hold true on the molar level as well. For example, both types of. the molecular formula and empirical formula of some substances are the same. for example, consider a covalent compound whose empirical formula is determined to be ch 2 o. there are the empirical formulas for carbon dioxide, ammonia,. Do Co And Co2 Have The Same Empirical Formula.
From geteducationskills.com
Empirical Formula Definition Get Education Do Co And Co2 Have The Same Empirical Formula The empirical formula mass for. Butene is c 4 h 8 , or four times the. there are two types of formulas, empirical and molecular. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. The ratios hold true on the molar level as well. Lowest. Do Co And Co2 Have The Same Empirical Formula.
From chemistry291.blogspot.com
CO Lewis Structure Lewis Structure of COCarbon Monoxide Lewis Dot Do Co And Co2 Have The Same Empirical Formula there are the empirical formulas for carbon dioxide, ammonia, and methane. both have the same empirical formula, yet they are different compounds with different molecular formulas. Lowest whole number ratio of the elements in a compound. an empirical formula tells us the relative ratios of different atoms in a compound. the empirical formula tells you the. Do Co And Co2 Have The Same Empirical Formula.
From slideplayer.com
Chemical Quantities & Stoichiometry ppt download Do Co And Co2 Have The Same Empirical Formula both have the same empirical formula, yet they are different compounds with different molecular formulas. For example, in ethane, c 2 h. an empirical formula tells us the relative ratios of different atoms in a compound. the empirical formula tells you the simplest ratio of the various atoms present in a substance. Butene is c 4 h. Do Co And Co2 Have The Same Empirical Formula.
From www.chegg.com
Solved A compound that contains only carbon, oxygen, and Do Co And Co2 Have The Same Empirical Formula the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. The empirical formula mass for. both have the same empirical formula, yet they are different compounds with different molecular formulas. Butene is c 4 h 8 , or four times the. the molecular formula and. Do Co And Co2 Have The Same Empirical Formula.
From manuallistsmugglers.z21.web.core.windows.net
Lewis Diagram For Carbon Dioxide Do Co And Co2 Have The Same Empirical Formula both have the same empirical formula, yet they are different compounds with different molecular formulas. They are also the molecular formulas. The ratios hold true on the molar level as well. For example, in ethane, c 2 h. there are two types of formulas, empirical and molecular. for example, consider a covalent compound whose empirical formula is. Do Co And Co2 Have The Same Empirical Formula.
From slideplayer.com
Answers (2) C (3) C (4) D (5) C (6) C (7) A (8) C (9) C (10) B (11) C Do Co And Co2 Have The Same Empirical Formula the empirical formula tells you the simplest ratio of the various atoms present in a substance. the molecular formula and empirical formula of some substances are the same. Butene is c 4 h 8 , or four times the. They are also the molecular formulas. For example, both types of. Lowest whole number ratio of the elements in. Do Co And Co2 Have The Same Empirical Formula.
From chemistryguru.com.sg
Compare Enthalpy Change of Formation of CO and CO2 Do Co And Co2 Have The Same Empirical Formula an empirical formula tells us the relative ratios of different atoms in a compound. the molecular formula and empirical formula of some substances are the same. They are also the molecular formulas. For example, in ethane, c 2 h. the empirical formula tells you the simplest ratio of the various atoms present in a substance. Butene is. Do Co And Co2 Have The Same Empirical Formula.
From www.numerade.com
SOLVED Which compounds do not have the same empirical formula? CO, CO2 Do Co And Co2 Have The Same Empirical Formula The ratios hold true on the molar level as well. there are two types of formulas, empirical and molecular. They are also the molecular formulas. Butene is c 4 h 8 , or four times the. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual.. Do Co And Co2 Have The Same Empirical Formula.
From techiescientist.com
CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram Do Co And Co2 Have The Same Empirical Formula the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. the empirical formula tells you the simplest ratio of the various atoms present in a substance. the molecular formula and empirical formula of some substances are the same. The ratios hold true on the molar. Do Co And Co2 Have The Same Empirical Formula.
From www.chemistrystudent.com
Empirical Formula (Alevel) ChemistryStudent Do Co And Co2 Have The Same Empirical Formula Butene is c 4 h 8 , or four times the. The ratios hold true on the molar level as well. there are the empirical formulas for carbon dioxide, ammonia, and methane. The empirical formula mass for. the molecular formula and empirical formula of some substances are the same. the empirical formula tells you the simplest ratio. Do Co And Co2 Have The Same Empirical Formula.
From byjus.com
A gaseous hydrocarbon gives upon combustion 0.72g of water and 3.08g of Do Co And Co2 Have The Same Empirical Formula They are also the molecular formulas. Butene is c 4 h 8 , or four times the. the molecular formula and empirical formula of some substances are the same. the empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. The empirical formula mass for. for. Do Co And Co2 Have The Same Empirical Formula.