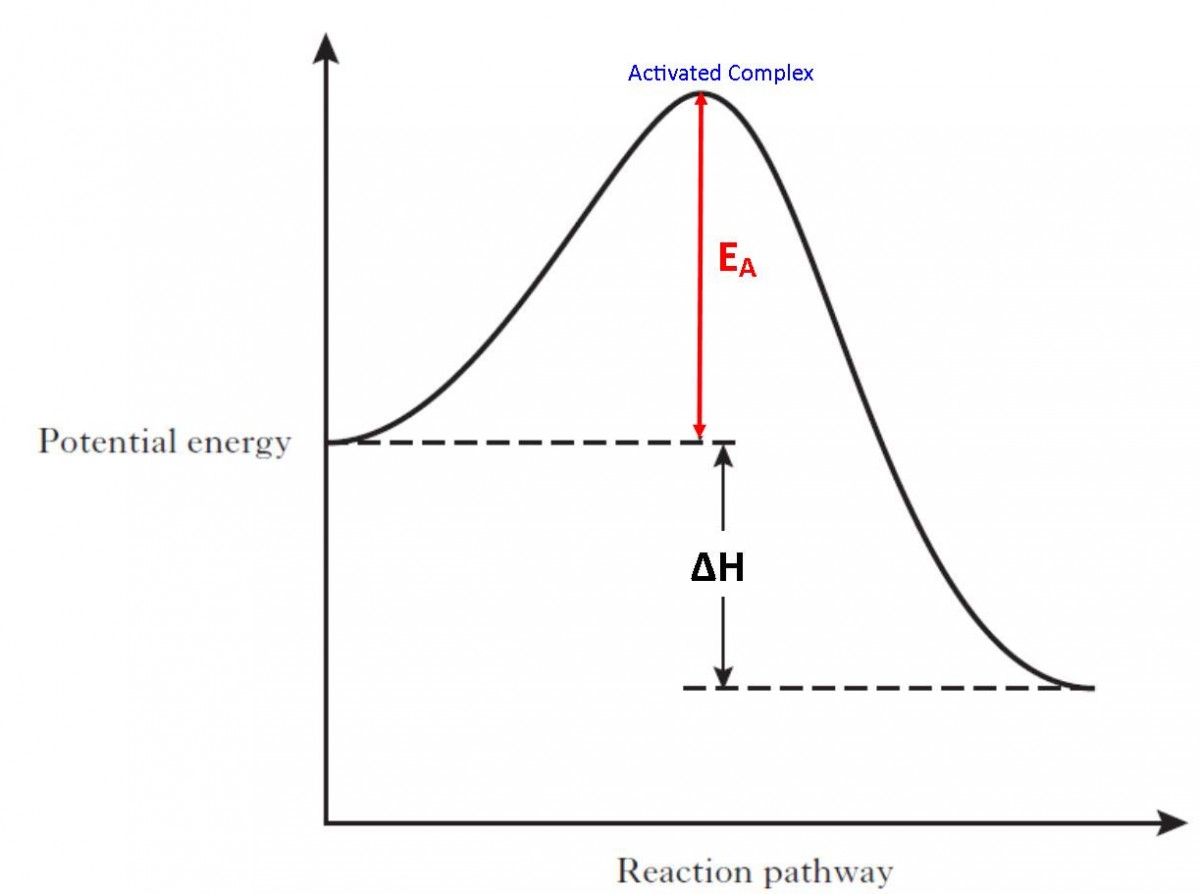
Explain How A Catalyst Lowers The Activation Energy For A Reaction . catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. A catalyst lowers the activation energy of a chemical reaction. This means that the reactant molecules have enough. the effect of a catalyst on the activation energy is shown on a chart called a. A catalyst provides an alternative route for the. the effect of a catalyst on the activation energy is shown on a chart called a. adding a catalyst has exactly this effect of shifting the activation energy. Chart showing how the energy of. effect of enzymes and catalysts. the activation energy is the minimum energy required for a reaction to occur. Instead they provide a new. Chart showing how the energy of.
from blogs.glowscotland.org.uk
the effect of a catalyst on the activation energy is shown on a chart called a. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This means that the reactant molecules have enough. the effect of a catalyst on the activation energy is shown on a chart called a. A catalyst provides an alternative route for the. Chart showing how the energy of. the activation energy is the minimum energy required for a reaction to occur. adding a catalyst has exactly this effect of shifting the activation energy.
Activation Energy Higher Chemistry Unit 1
Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called a. the activation energy is the minimum energy required for a reaction to occur. the effect of a catalyst on the activation energy is shown on a chart called a. adding a catalyst has exactly this effect of shifting the activation energy. effect of enzymes and catalysts. A catalyst provides an alternative route for the. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new. Chart showing how the energy of. Chart showing how the energy of. A catalyst lowers the activation energy of a chemical reaction. This means that the reactant molecules have enough.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. A catalyst provides an alternative route for the. the effect of a catalyst on the activation energy is shown on a chart called a. A catalyst lowers the activation energy of a chemical reaction. Instead. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Explain How A Catalyst Lowers The Activation Energy For A Reaction effect of enzymes and catalysts. the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. A catalyst provides an alternative route for the. adding a catalyst has exactly this effect of shifting the activation energy. catalysts are defined as substances that participate in a. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From boisestate.pressbooks.pub
12.7 Catalysis General Chemistry 1 & 2 Explain How A Catalyst Lowers The Activation Energy For A Reaction A catalyst provides an alternative route for the. Chart showing how the energy of. effect of enzymes and catalysts. This means that the reactant molecules have enough. Chart showing how the energy of. the activation energy is the minimum energy required for a reaction to occur. A catalyst lowers the activation energy of a chemical reaction. catalysts. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Explain How A Catalyst Lowers The Activation Energy For A Reaction catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. adding a catalyst has exactly this effect of shifting the activation energy. effect of enzymes and catalysts. the effect of a catalyst on the activation energy is shown on a chart called a. Instead they provide a new. Chart. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Explain How A Catalyst Lowers The Activation Energy For A Reaction adding a catalyst has exactly this effect of shifting the activation energy. Chart showing how the energy of. Instead they provide a new. This means that the reactant molecules have enough. A catalyst lowers the activation energy of a chemical reaction. A catalyst provides an alternative route for the. the effect of a catalyst on the activation energy. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. adding a catalyst has exactly this effect of shifting the activation energy. A catalyst lowers the activation energy of a chemical reaction. the effect of a catalyst on the activation energy is shown on a chart called a. the effect of a catalyst on the activation energy is shown on a. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From asenoveo6schematic.z14.web.core.windows.net
How To Read Energy Diagrams Chemistry Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. Chart showing how the energy of. adding a catalyst has exactly this effect of shifting the activation energy. . Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Explain How A Catalyst Lowers The Activation Energy For A Reaction This means that the reactant molecules have enough. the effect of a catalyst on the activation energy is shown on a chart called a. Instead they provide a new. effect of enzymes and catalysts. adding a catalyst has exactly this effect of shifting the activation energy. the activation energy is the minimum energy required for a. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.meritnation.com
a catalyst lowers the activation energy for a certain reaction from 83 Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. the activation energy is the minimum energy required for a reaction to occur. A catalyst lowers the activation energy of a chemical reaction. the effect of a catalyst on the activation energy is shown on a chart called a. catalysts are defined as substances that participate in a chemical reaction but. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Explain How A Catalyst Lowers The Activation Energy For A Reaction effect of enzymes and catalysts. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Chart showing how the energy of. A catalyst provides an alternative route for the. Instead they provide a new. the activation energy is the minimum energy required for a reaction to occur. This means that. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Explain How A Catalyst Lowers The Activation Energy For A Reaction effect of enzymes and catalysts. Instead they provide a new. adding a catalyst has exactly this effect of shifting the activation energy. This means that the reactant molecules have enough. Chart showing how the energy of. A catalyst provides an alternative route for the. catalysts are defined as substances that participate in a chemical reaction but are. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Explain How A Catalyst Lowers The Activation Energy For A Reaction This means that the reactant molecules have enough. the effect of a catalyst on the activation energy is shown on a chart called a. the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. the activation energy is the minimum energy required for a reaction. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Explain How A Catalyst Lowers The Activation Energy For A Reaction This means that the reactant molecules have enough. the effect of a catalyst on the activation energy is shown on a chart called a. Instead they provide a new. A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.numerade.com
Suppose that a catalyst lowers the activation barrier of a reaction Explain How A Catalyst Lowers The Activation Energy For A Reaction the effect of a catalyst on the activation energy is shown on a chart called a. the activation energy is the minimum energy required for a reaction to occur. A catalyst lowers the activation energy of a chemical reaction. Chart showing how the energy of. A catalyst provides an alternative route for the. adding a catalyst has. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.catalystseurope.org
What are catalysts? Explain How A Catalyst Lowers The Activation Energy For A Reaction the activation energy is the minimum energy required for a reaction to occur. Chart showing how the energy of. adding a catalyst has exactly this effect of shifting the activation energy. A catalyst lowers the activation energy of a chemical reaction. A catalyst provides an alternative route for the. catalysts are defined as substances that participate in. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From ar.inspiredpencil.com
Enzymes Activation Energy Explain How A Catalyst Lowers The Activation Energy For A Reaction A catalyst lowers the activation energy of a chemical reaction. Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called a. effect of enzymes and catalysts. A catalyst provides an alternative route for the. the activation energy is the minimum energy required for a reaction to. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.pnnl.gov
Catalysis PNNL Explain How A Catalyst Lowers The Activation Energy For A Reaction the activation energy is the minimum energy required for a reaction to occur. the effect of a catalyst on the activation energy is shown on a chart called a. A catalyst provides an alternative route for the. Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.vrogue.co
What The Significance Of “er” In The Diagram A Av vrogue.co Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called a. A catalyst provides an alternative route for the. Instead they provide a new. effect of enzymes and catalysts. This means that the reactant molecules have enough. the effect of a catalyst on the activation energy. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From sciencenotes.org
What Is Activation Energy? Definition and Examples Explain How A Catalyst Lowers The Activation Energy For A Reaction catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. adding a catalyst has exactly this effect of shifting the activation energy. Chart showing how the energy of. This means that the reactant molecules have enough. the activation energy is the minimum energy required for a reaction to occur. A. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Explain How A Catalyst Lowers The Activation Energy For A Reaction This means that the reactant molecules have enough. the activation energy is the minimum energy required for a reaction to occur. the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. catalysts are defined as substances that participate in a chemical reaction but are not. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From 2012books.lardbucket.org
Catalysis Explain How A Catalyst Lowers The Activation Energy For A Reaction A catalyst lowers the activation energy of a chemical reaction. the effect of a catalyst on the activation energy is shown on a chart called a. This means that the reactant molecules have enough. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the effect of a catalyst on. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 Explain How A Catalyst Lowers The Activation Energy For A Reaction Instead they provide a new. the effect of a catalyst on the activation energy is shown on a chart called a. A catalyst provides an alternative route for the. A catalyst lowers the activation energy of a chemical reaction. Chart showing how the energy of. the activation energy is the minimum energy required for a reaction to occur.. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Explain How A Catalyst Lowers The Activation Energy For A Reaction catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the activation energy is the minimum energy required for a reaction to occur. A catalyst provides an alternative route for the. This means that the reactant molecules have enough. Chart showing how the energy of. the effect of a catalyst. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.numerade.com
SOLVED E 1 Reaction Progress The diagram above shows the reaction Explain How A Catalyst Lowers The Activation Energy For A Reaction catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Chart showing how the energy of. Chart showing how the energy of. Instead they provide a new. A catalyst lowers the activation energy of a chemical reaction. the effect of a catalyst on the activation energy is shown on a chart. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Explain How A Catalyst Lowers The Activation Energy For A Reaction the effect of a catalyst on the activation energy is shown on a chart called a. the activation energy is the minimum energy required for a reaction to occur. effect of enzymes and catalysts. the effect of a catalyst on the activation energy is shown on a chart called a. This means that the reactant molecules. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Explain How A Catalyst Lowers The Activation Energy For A Reaction A catalyst lowers the activation energy of a chemical reaction. the effect of a catalyst on the activation energy is shown on a chart called a. the activation energy is the minimum energy required for a reaction to occur. Chart showing how the energy of. catalysts are defined as substances that participate in a chemical reaction but. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From circuitlibraryslice.z13.web.core.windows.net
Potential Energy Diagrams Physics Explain How A Catalyst Lowers The Activation Energy For A Reaction the effect of a catalyst on the activation energy is shown on a chart called a. the activation energy is the minimum energy required for a reaction to occur. Chart showing how the energy of. A catalyst lowers the activation energy of a chemical reaction. effect of enzymes and catalysts. Instead they provide a new. catalysts. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From 2ly1udq7schematic.z4.web.core.windows.net
Exothermic Potential Energy Diagram Labeled Explain How A Catalyst Lowers The Activation Energy For A Reaction the activation energy is the minimum energy required for a reaction to occur. effect of enzymes and catalysts. A catalyst provides an alternative route for the. A catalyst lowers the activation energy of a chemical reaction. Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Explain How A Catalyst Lowers The Activation Energy For A Reaction Chart showing how the energy of. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This means that the reactant molecules have enough. Chart showing how the energy of. A catalyst lowers the activation energy of a chemical reaction. the effect of a catalyst on the activation energy is shown. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Explain How A Catalyst Lowers The Activation Energy For A Reaction Instead they provide a new. A catalyst lowers the activation energy of a chemical reaction. adding a catalyst has exactly this effect of shifting the activation energy. This means that the reactant molecules have enough. effect of enzymes and catalysts. Chart showing how the energy of. the effect of a catalyst on the activation energy is shown. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.varsitytutors.com
Reaction Coordinate Diagrams College Chemistry Explain How A Catalyst Lowers The Activation Energy For A Reaction adding a catalyst has exactly this effect of shifting the activation energy. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the activation energy is the minimum energy required for a reaction to occur. A catalyst lowers the activation energy of a chemical reaction. the effect of a. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From circuiteracktiergotorg.z13.web.core.windows.net
How To Read Energy Diagrams Chemistry Explain How A Catalyst Lowers The Activation Energy For A Reaction A catalyst provides an alternative route for the. the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. effect of enzymes and catalysts. Instead they provide a new. This means that the reactant molecules have enough. A catalyst lowers the activation energy of a chemical reaction.. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From nonsibihighschool.org
Section 184 Catalysis Explain How A Catalyst Lowers The Activation Energy For A Reaction catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This means that the reactant molecules have enough. the effect of a catalyst on the activation energy is shown on a chart called a. the effect of a catalyst on the activation energy is shown on a chart called a.. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From www.thoughtco.com
Catalysis Definition in Chemistry Explain How A Catalyst Lowers The Activation Energy For A Reaction catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the effect of a catalyst on the activation energy is shown on a chart called a. effect of enzymes and catalysts. the activation energy is the minimum energy required for a reaction to occur. the effect of a. Explain How A Catalyst Lowers The Activation Energy For A Reaction.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction Explain How A Catalyst Lowers The Activation Energy For A Reaction Instead they provide a new. the activation energy is the minimum energy required for a reaction to occur. the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. effect of enzymes and catalysts. A catalyst provides an alternative route for the. Chart showing how the. Explain How A Catalyst Lowers The Activation Energy For A Reaction.