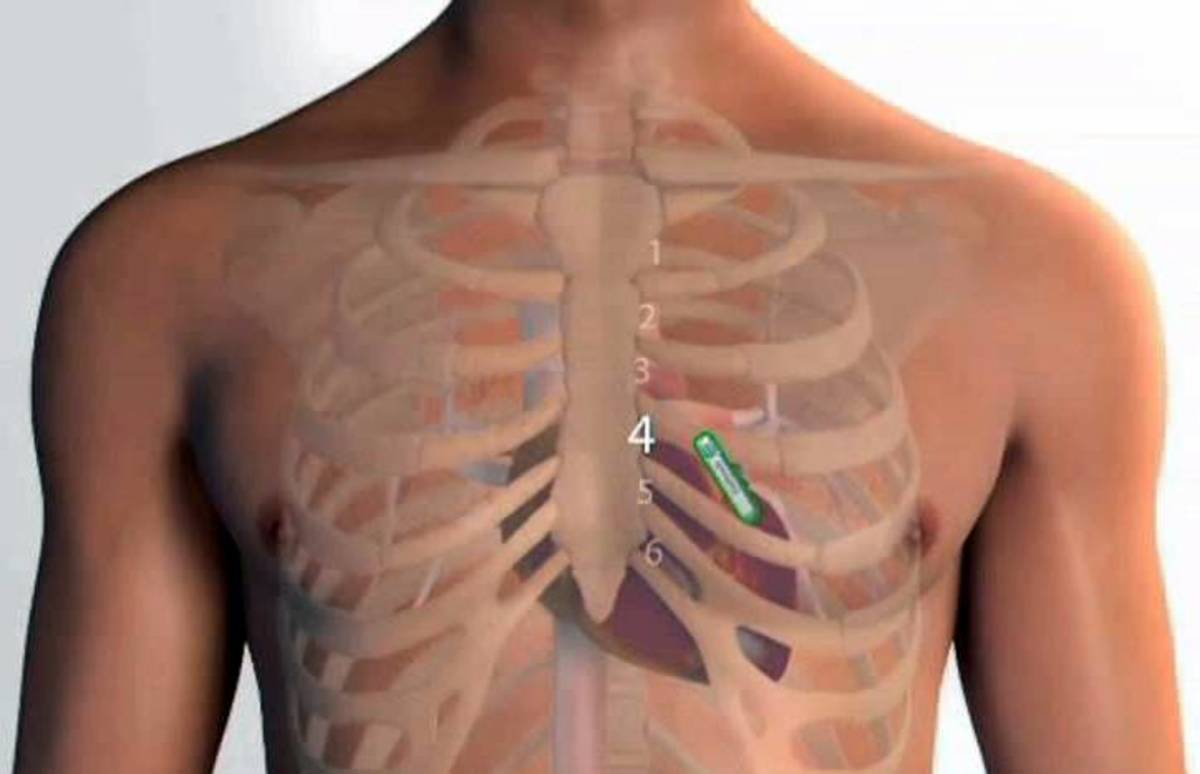
Medical Device Implant Card . according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. You will be given a temporary medical. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. in an emergency, the card will alert medical and security personnel that you have an implanted device.
from patientslounge.com
Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. You will be given a temporary medical. in an emergency, the card will alert medical and security personnel that you have an implanted device. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those.
The Implantable Medtronic Cardiac Loop Recorder A Personal Experience
Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. You will be given a temporary medical. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. in an emergency, the card will alert medical and security personnel that you have an implanted device. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios.
From www.youtube.com
Getting an insertable cardiac monitor (loop recorder)? Watch an implant Medical Device Implant Card You will be given a temporary medical. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. in an emergency, the card will alert medical and security personnel that you have an implanted device. according to. Medical Device Implant Card.
From www.ibi-sa.com
Implant card IBI SA Medical Device Implant Card They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. in an emergency, the card will alert medical and security personnel that you have an implanted device. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. You will be given a temporary medical. according to. Medical Device Implant Card.
From www.pinterest.com
the m t2 logo is shown in blue and white, as well as an image of Medical Device Implant Card You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. in an emergency, the card will alert medical and security personnel that you have an implanted device. Implant cards are required under article 18 of eu regulation 2017/745 on medical. Medical Device Implant Card.
From integrum.se
Implant Card Integrum Medical Device Implant Card They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. in an emergency, the card will alert medical and security personnel that you have an implanted device. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. You will. Medical Device Implant Card.
From www.jnjmedtech.com
LINX® Imaging for Diagnosis by ETHICON™ J&J MedTech Medical Device Implant Card You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. . Medical Device Implant Card.
From www.qualitymeddev.com
Implantable Medical Devices and related EU MDR Requirements Medical Device Implant Card Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. in an emergency, the card. Medical Device Implant Card.
From www.mergemed.com
Implants Merege Medical Device Studio Medical Device Implant Card in an emergency, the card will alert medical and security personnel that you have an implanted device. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. You will be given a temporary medical. this factsheet. Medical Device Implant Card.
From www.biotronik.com
International Implant Card Biotronik Medical Device Implant Card Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential information about their. Medical Device Implant Card.
From www.pinterest.com
implant card design for VasQ Medical terms, Medical, Card design Medical Device Implant Card Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. in an emergency, the card will alert medical and security personnel that you have an implanted. Medical Device Implant Card.
From www.bonalive.com
Patient information Bonalive Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. in an emergency, the card will alert medical and security personnel that you have an implanted device. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. according to article 18 of regulation (eu) 2017/745 on medical devices,. Medical Device Implant Card.
From heartbeataz.com
What You Need to Know About the Implantable Cardioverter Defibrillator Medical Device Implant Card They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. according to article 18 of regulation (eu) 2017/745 on medical devices,. Medical Device Implant Card.
From mdlaw.eu
Manufacturers of implantable medical devices new MDCG guidance on Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. according to article 18 of regulation (eu) 2017/745 on medical devices,. Medical Device Implant Card.
From www.bostonscientific.com
Recovering from your procedure Boston Scientific Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. in an emergency, the card will alert. Medical Device Implant Card.
From www.gomaro.ch
Carte d'implant Implant Card Gomaro s.a. Medical Device Implant Card in an emergency, the card will alert medical and security personnel that you have an implanted device. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. You will be given a temporary medical. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. according to. Medical Device Implant Card.
From 13485.info
MDR’ın İmplant Kartı Gereklilikleri ConsulTeam Medical Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. in an emergency, the card will alert medical and security personnel that you have an implanted device. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. this. Medical Device Implant Card.
From exopzcrah.blob.core.windows.net
Implant Identification Card at Nicole Yetter blog Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. in an emergency, the card will alert medical and security personnel that you have an implanted device. You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to. Medical Device Implant Card.
From www.vistaar.ai
Medical Device Patient Information Leaflets and Implant Cards Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. in an emergency, the card will alert medical and security personnel that you have. Medical Device Implant Card.
From www.wevolver.com
Custom 3D Printed Medical Devices Trends and Opportunities Medical Device Implant Card in an emergency, the card will alert medical and security personnel that you have an implanted device. You will be given a temporary medical. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential. Medical Device Implant Card.
From www.slideshare.net
Presentation MMDR reform Patient Implant Cards and Information Lea… Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. You will be given a temporary medical. in an emergency, the card will alert medical and security personnel that. Medical Device Implant Card.
From www.jnjmedtech.com
LINX® Imaging for Diagnosis Ethicon Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. You will be given a temporary medical. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. according to article 18. Medical Device Implant Card.
From www.stevenlabel.com
Medical Device Mounting Cards Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. You will be given a temporary medical. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. Implant cards are required under article 18 of eu regulation 2017/745 on medical. Medical Device Implant Card.
From dxoindfjv.blob.core.windows.net
Implant Card For Medical Devices at Betty Gray blog Medical Device Implant Card They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. You will be given. Medical Device Implant Card.
From operonstrategist.com
Comprehensive Guide to Active Implantable Medical Devices (AIMD Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. in an emergency, the card will alert medical and security personnel that you have an implanted device. according to article 18 of regulation (eu) 2017/745 on medical devices,. Medical Device Implant Card.
From syntropiq.com
Patient Implant Info Syntropiq Medical Device Implant Card They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt). Medical Device Implant Card.
From www.ibi-sa.com
Implant card IBI SA Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. in an emergency, the card will alert medical and security personnel that you have an implanted device. You will. Medical Device Implant Card.
From patientslounge.com
The Implantable Medtronic Cardiac Loop Recorder A Personal Experience Medical Device Implant Card in an emergency, the card will alert medical and security personnel that you have an implanted device. You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. Implant cards are required under article 18 of eu regulation 2017/745 on medical. Medical Device Implant Card.
From integrum.se
Implant Card Integrum Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. You will be given. Medical Device Implant Card.
From integrum.se
Implant Card Integrum Medical Device Implant Card You will be given a temporary medical. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential information about their implanted medical devices and improve patient safety. Medical Device Implant Card.
From www.singhealth.com.sg
Next generation cardiac implantable electronic devices SingHealth Medical Device Implant Card in an emergency, the card will alert medical and security personnel that you have an implanted device. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. You will be given a temporary medical. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. Implant. Medical Device Implant Card.
From www.youtube.com
Patient Implant Cards YouTube Medical Device Implant Card according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. You will be given a temporary medical. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. in an emergency, the card will alert medical and security personnel that you have an implanted. Medical Device Implant Card.
From omcmedical.com
Implant Card for Switzerland OMC Medical Medical Device Implant Card Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. You will be given a temporary medical. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. according to article 18. Medical Device Implant Card.
From www.cookmedical.eu
Cook Medical and EU MDR Cook Medical Medical Device Implant Card They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. according to article 18 of regulation (eu) 2017/745 on medical devices, the european union (eu) requires manufacturers to provide “implant cards”. Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. in an emergency, the card. Medical Device Implant Card.
From youregulate.com
Guidance on Implant Card Medical Device Implant Card Implant cards are required under article 18 of eu regulation 2017/745 on medical devices. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. according to article 18 of regulation (eu) 2017/745 on medical devices,. Medical Device Implant Card.
From www.ota.org.au
News TGA Medical Device patient Information leaflets and information Medical Device Implant Card in an emergency, the card will alert medical and security personnel that you have an implanted device. You will be given a temporary medical. this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. . Medical Device Implant Card.
From mavenprofserv.com
Implant Card for Medical Device Your Complete Guide Medical Device Implant Card this factsheet explains implant card requirements, how johnson & johnson medtech (jjmt) is fulfilling those. You will be given a temporary medical. They provide patients with essential information about their implanted medical devices and improve patient safety in various scenarios. in an emergency, the card will alert medical and security personnel that you have an implanted device. . Medical Device Implant Card.