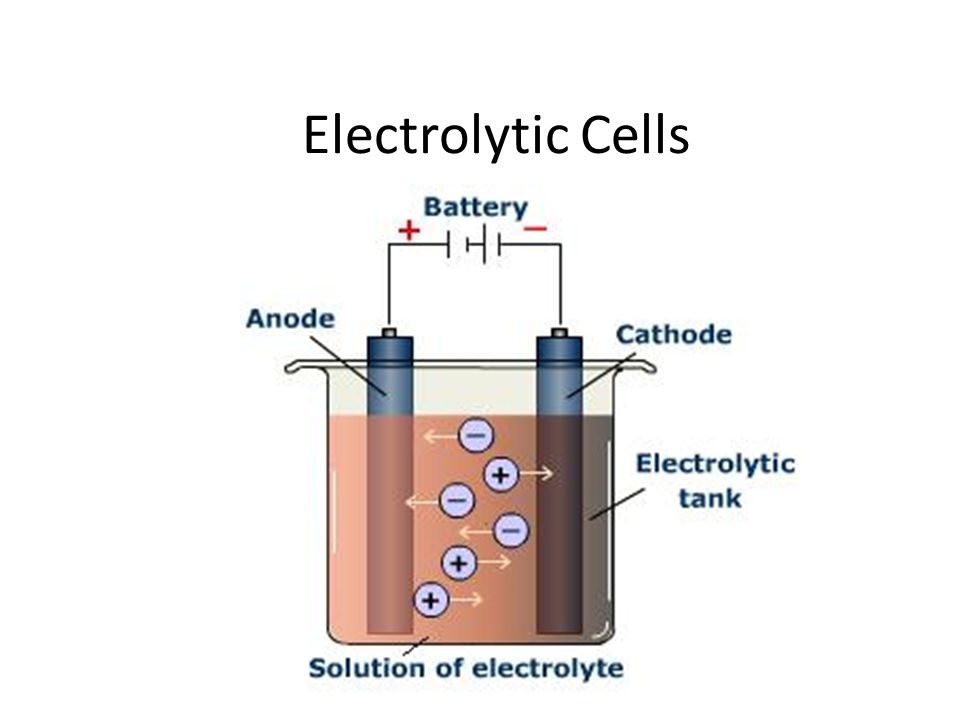
How Many Electrodes Are There In Electrolysis . electrolysis involves using electricity to break down electrolytes to form elements. By using direct electrical currents, we can split up ionic compounds into their positive and. electrolysis splits up ionic compounds. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. In this chapter, we have described various galvanic cells in which. to understand electrolysis and describe it quantitatively. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. The products of electrolysis can be. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. These electrodes are often made of an.
from circuitlibrarybauer.z13.web.core.windows.net
By using direct electrical currents, we can split up ionic compounds into their positive and. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. These electrodes are often made of an. The products of electrolysis can be. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. to understand electrolysis and describe it quantitatively. electrolysis involves using electricity to break down electrolytes to form elements. electrolysis splits up ionic compounds. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis.
Strong Electrolyte Diagram
How Many Electrodes Are There In Electrolysis An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. These electrodes are often made of an. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. By using direct electrical currents, we can split up ionic compounds into their positive and. electrolysis involves using electricity to break down electrolytes to form elements. to understand electrolysis and describe it quantitatively. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. In this chapter, we have described various galvanic cells in which. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. electrolysis splits up ionic compounds. The products of electrolysis can be.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 How Many Electrodes Are There In Electrolysis These electrodes are often made of an. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. In this chapter, we have described various galvanic cells in which. electrolysis splits up ionic compounds. electrolysis involves using electricity to break down electrolytes to form elements. to understand electrolysis and describe. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte How Many Electrodes Are There In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. to understand electrolysis and describe it quantitatively. In this chapter, we have described various galvanic cells in which. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. These electrodes are often made of an. By using direct. How Many Electrodes Are There In Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry How Many Electrodes Are There In Electrolysis In this chapter, we have described various galvanic cells in which. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. electrolysis splits up ionic compounds. to understand electrolysis and describe it quantitatively.. How Many Electrodes Are There In Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 How Many Electrodes Are There In Electrolysis The products of electrolysis can be. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. In this chapter, we have described various galvanic cells in which. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. These electrodes are often. How Many Electrodes Are There In Electrolysis.
From mungfali.com
Electrolysis Process Diagram How Many Electrodes Are There In Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. These electrodes are often made of an. In this chapter, we have described various galvanic cells in which. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. the electrode attached to the negative terminal. How Many Electrodes Are There In Electrolysis.
From rodneyyouthmata.blogspot.com
Anode and Cathode in Electrolysis How Many Electrodes Are There In Electrolysis electrolysis splits up ionic compounds. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The products of electrolysis can be. By using direct electrical currents, we can split up. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download How Many Electrodes Are There In Electrolysis the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. to understand electrolysis and describe it quantitatively. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. These electrodes are often made of an. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process. How Many Electrodes Are There In Electrolysis.
From www.science-revision.co.uk
Cells and batteries How Many Electrodes Are There In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. In this chapter, we have described various galvanic cells in which. By using direct electrical currents, we can split up ionic compounds into their positive and. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 How Many Electrodes Are There In Electrolysis electrolysis splits up ionic compounds. to understand electrolysis and describe it quantitatively. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins. How Many Electrodes Are There In Electrolysis.
From userdiagramkipped.z21.web.core.windows.net
Picture Of Anode Electrolyte Cathode In Cell How Many Electrodes Are There In Electrolysis electrolysis splits up ionic compounds. to understand electrolysis and describe it quantitatively. These electrodes are often made of an. In this chapter, we have described various galvanic cells in which. By using direct electrical currents, we can split up ionic compounds into their positive and. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed.. How Many Electrodes Are There In Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science How Many Electrodes Are There In Electrolysis The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form elements. In this chapter, we have described various galvanic cells in which. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An ionic compound occurs when a negative ion (an atom that has gained. How Many Electrodes Are There In Electrolysis.
From exowuasqn.blob.core.windows.net
Stainless Steel Electrodes For Electrolysis at Loretta Bryan blog How Many Electrodes Are There In Electrolysis By using direct electrical currents, we can split up ionic compounds into their positive and. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. to understand electrolysis and describe it quantitatively. These electrodes are often made of an. In this chapter, we have described various galvanic cells in which. two. How Many Electrodes Are There In Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic How Many Electrodes Are There In Electrolysis electrolysis splits up ionic compounds. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. electrolysis involves using electricity to break down electrolytes to form elements. to understand electrolysis and describe it quantitatively. These electrodes are often made of an. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process. How Many Electrodes Are There In Electrolysis.
From www.vrogue.co
Introduction To Electrolysis Redox Reactions And Elec vrogue.co How Many Electrodes Are There In Electrolysis electrolysis splits up ionic compounds. to understand electrolysis and describe it quantitatively. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. In this chapter, we have described various galvanic cells in which. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a. How Many Electrodes Are There In Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry How Many Electrodes Are There In Electrolysis An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. electrolysis involves using electricity to break down electrolytes to form elements. to understand electrolysis and describe it quantitatively. These electrodes are often made of an. In this chapter, we have described various galvanic cells in which.. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 How Many Electrodes Are There In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. By using direct electrical currents, we can split up ionic compounds into their positive and. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. two electrical conductors (electrodes) are. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 How Many Electrodes Are There In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The products of electrolysis can be. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. electrolysis involves using electricity to break down electrolytes to form elements. electrolysis splits up ionic compounds. In. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download How Many Electrodes Are There In Electrolysis By using direct electrical currents, we can split up ionic compounds into their positive and. In this chapter, we have described various galvanic cells in which. electrolysis splits up ionic compounds. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The products of electrolysis can be. These electrodes are often made. How Many Electrodes Are There In Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes How Many Electrodes Are There In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The products of electrolysis can be. These electrodes are often made of an. electrolysis involves using electricity to break down electrolytes to form elements. An ionic compound occurs. How Many Electrodes Are There In Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki How Many Electrodes Are There In Electrolysis By using direct electrical currents, we can split up ionic compounds into their positive and. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An ionic compound occurs when a negative ion (an atom that has gained an. How Many Electrodes Are There In Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog How Many Electrodes Are There In Electrolysis The products of electrolysis can be. These electrodes are often made of an. In this chapter, we have described various galvanic cells in which. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. By using direct electrical currents,. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS OF AQUEOUS SOLUTIONS PowerPoint Presentation, free How Many Electrodes Are There In Electrolysis to understand electrolysis and describe it quantitatively. These electrodes are often made of an. The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form elements. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. electrolysis splits up ionic compounds. In this chapter, we have described various galvanic. How Many Electrodes Are There In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Many Electrodes Are There In Electrolysis By using direct electrical currents, we can split up ionic compounds into their positive and. electrolysis involves using electricity to break down electrolytes to form elements. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. The products of electrolysis can be. An ionic compound occurs when a negative ion (an atom that has gained an. How Many Electrodes Are There In Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis How Many Electrodes Are There In Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. These electrodes are often made of an. By using direct electrical currents, we can split up ionic compounds. How Many Electrodes Are There In Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS How Many Electrodes Are There In Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. to understand electrolysis and describe it quantitatively. These electrodes are often made of an. in electrolytic cells, electrical. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 How Many Electrodes Are There In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form elements. in electrolytic cells, electrical energy causes. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID3366458 How Many Electrodes Are There In Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. By using direct electrical currents, we can split up ionic compounds into their positive and. to understand electrolysis and describe it quantitatively. In this chapter, we have described various galvanic cells in which. the electrode attached to the negative terminal. How Many Electrodes Are There In Electrolysis.
From circuitagolopetzin.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis How Many Electrodes Are There In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form elements. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. In this chapter, we have described various galvanic cells in which. These. How Many Electrodes Are There In Electrolysis.
From ar.inspiredpencil.com
Electrolyte Charting Diagram How Many Electrodes Are There In Electrolysis In this chapter, we have described various galvanic cells in which. to understand electrolysis and describe it quantitatively. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. These. How Many Electrodes Are There In Electrolysis.
From en.ppt-online.org
Electrolysis online presentation How Many Electrodes Are There In Electrolysis The products of electrolysis can be. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. electrolysis involves using electricity to break down electrolytes to form elements. to understand electrolysis and describe it quantitatively. electrolysis splits up ionic compounds. in electrolytic cells, electrical energy causes nonspontaneous reactions to. How Many Electrodes Are There In Electrolysis.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students How Many Electrodes Are There In Electrolysis These electrodes are often made of an. By using direct electrical currents, we can split up ionic compounds into their positive and. The products of electrolysis can be. to understand electrolysis and describe it quantitatively. In this chapter, we have described various galvanic cells in which. An ionic compound occurs when a negative ion (an atom that has gained. How Many Electrodes Are There In Electrolysis.
From www.slideserve.com
PPT 17.78 Electrolysis & Applications PowerPoint Presentation ID How Many Electrodes Are There In Electrolysis These electrodes are often made of an. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. electrolysis involves using electricity to break down electrolytes to form elements. In this chapter, we have described various galvanic cells in which. By using direct electrical currents, we can split up ionic compounds into their positive and. the. How Many Electrodes Are There In Electrolysis.
From circuitlibrarybauer.z13.web.core.windows.net
Strong Electrolyte Diagram How Many Electrodes Are There In Electrolysis These electrodes are often made of an. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. electrolysis splits up ionic compounds. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. two electrical conductors (electrodes) are immersed in. How Many Electrodes Are There In Electrolysis.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse How Many Electrodes Are There In Electrolysis These electrodes are often made of an. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. electrolysis involves using electricity to break down electrolytes to form elements. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. In. How Many Electrodes Are There In Electrolysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts How Many Electrodes Are There In Electrolysis The products of electrolysis can be. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. These electrodes are often made of an. In this chapter, we have described various. How Many Electrodes Are There In Electrolysis.