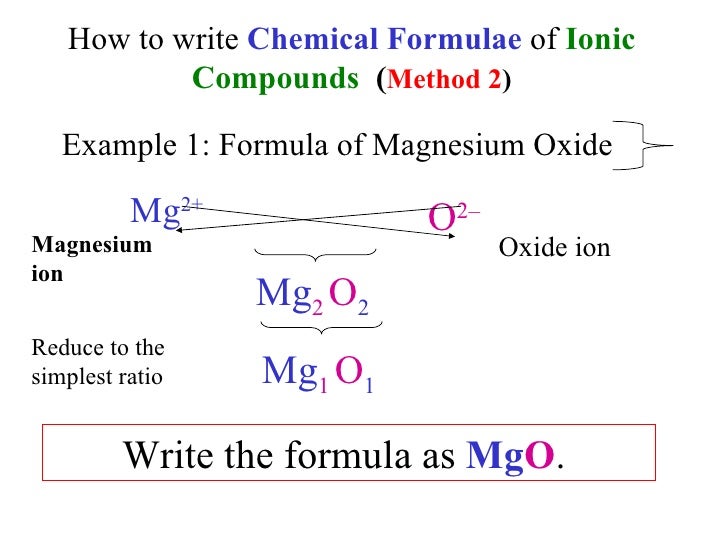
Oxygen Magnesium Chloride Chemical Formula . when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. Butane, which has the chemical formula c₄h₁₀, has four carbon. magnesium chloride + oxygen produces magnesium chlorate. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. what is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. This shows that it has. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. for example, magnesium oxide is made up of two elements, magnesium and oxygen. Magnesium chloride is an ionic.
from www.slideshare.net
write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. what is the formula for barium chloride? magnesium chloride + oxygen produces magnesium chlorate. This shows that it has. for example, magnesium oxide is made up of two elements, magnesium and oxygen. Magnesium chloride is an ionic. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Butane, which has the chemical formula c₄h₁₀, has four carbon. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and.
Chem matters ch6_ionic_bond
Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. for example, magnesium oxide is made up of two elements, magnesium and oxygen. Butane, which has the chemical formula c₄h₁₀, has four carbon. Magnesium chloride is an ionic. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. magnesium chloride + oxygen produces magnesium chlorate. what is the formula for barium chloride? when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. This shows that it has.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Oxygen Magnesium Chloride Chemical Formula Magnesium chloride is an ionic. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. magnesium chloride + oxygen produces magnesium chlorate. write. Oxygen Magnesium Chloride Chemical Formula.
From www.knowledgeboat.com
Explain with the help of (i) an ionic equation (ii) KnowledgeBoat Oxygen Magnesium Chloride Chemical Formula Magnesium chloride is an ionic. This shows that it has. magnesium chloride + oxygen produces magnesium chlorate. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. for example, magnesium oxide is made up of two elements, magnesium and oxygen. what. Oxygen Magnesium Chloride Chemical Formula.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Oxygen Magnesium Chloride Chemical Formula the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. This shows that it has. magnesium chloride + oxygen produces. Oxygen Magnesium Chloride Chemical Formula.
From galvinconanstuart.blogspot.com
Dot Diagram Of Magnesium Chloride General Wiring Diagram Oxygen Magnesium Chloride Chemical Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. magnesium chloride + oxygen produces magnesium chlorate. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. what is the formula for barium chloride? This shows. Oxygen Magnesium Chloride Chemical Formula.
From www.tessshebaylo.com
Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen Oxygen Magnesium Chloride Chemical Formula This shows that it has. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. Butane, which has the chemical formula c₄h₁₀, has four carbon. what. Oxygen Magnesium Chloride Chemical Formula.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. This shows that it has. for example, magnesium oxide is made up of two elements, magnesium and oxygen. Butane, which has the chemical formula c₄h₁₀, has four carbon. what is the formula. Oxygen Magnesium Chloride Chemical Formula.
From www.alamy.com
3D image of Magnesium chloride skeletal formula molecular chemical Oxygen Magnesium Chloride Chemical Formula This shows that it has. Butane, which has the chemical formula c₄h₁₀, has four carbon. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. Magnesium chloride is an ionic. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one. Oxygen Magnesium Chloride Chemical Formula.
From www.vrogue.co
Chemical Formula Of The Following Compounds By Crissc vrogue.co Oxygen Magnesium Chloride Chemical Formula This shows that it has. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. for example, magnesium oxide is. Oxygen Magnesium Chloride Chemical Formula.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. magnesium chloride + oxygen produces magnesium chlorate. Magnesium chloride is an ionic.. Oxygen Magnesium Chloride Chemical Formula.
From wiredatapoliciaj0.z22.web.core.windows.net
Mg Dot Diagram Oxygen Magnesium Chloride Chemical Formula Butane, which has the chemical formula c₄h₁₀, has four carbon. what is the formula for barium chloride? the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. magnesium chloride + oxygen produces magnesium chlorate. write the chemical formula for the compound that is formed. Oxygen Magnesium Chloride Chemical Formula.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Oxygen Magnesium Chloride Chemical Formula Magnesium chloride is an ionic. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Butane, which has the chemical formula c₄h₁₀, has four carbon. magnesium chloride + oxygen produces magnesium chlorate. the chemical formula for magnesium oxide, mgo, tells us that there. Oxygen Magnesium Chloride Chemical Formula.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Oxygen Magnesium Chloride Chemical Formula This shows that it has. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. magnesium chloride + oxygen produces magnesium chlorate.. Oxygen Magnesium Chloride Chemical Formula.
From www.chegg.com
REPORT SHEET LAB Chemical Reactions and Equations 8 Oxygen Magnesium Chloride Chemical Formula the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. This shows that it has. Magnesium chloride is an ionic. when an ionic compound is formed. Oxygen Magnesium Chloride Chemical Formula.
From www.showme.com
Formula for Magnesium oxide Science, Chemistry ShowMe Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. for example, magnesium oxide is made up of. Oxygen Magnesium Chloride Chemical Formula.
From testbook.com
Magnesium Chloride Preparation, Structure, Formula, Properties Oxygen Magnesium Chloride Chemical Formula Butane, which has the chemical formula c₄h₁₀, has four carbon. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. This shows that it has. magnesium chloride + oxygen produces magnesium chlorate. for example, magnesium oxide is made up of two elements,. Oxygen Magnesium Chloride Chemical Formula.
From exolrsqck.blob.core.windows.net
Magnesium Chloride Dot And Cross at Florence Bottoms blog Oxygen Magnesium Chloride Chemical Formula This shows that it has. Magnesium chloride is an ionic. for example, magnesium oxide is made up of two elements, magnesium and oxygen. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. the chemical formula for magnesium oxide, mgo, tells us. Oxygen Magnesium Chloride Chemical Formula.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Reaction Between Magnesium And Oxygen Gas Oxygen Magnesium Chloride Chemical Formula Magnesium chloride is an ionic. what is the formula for barium chloride? This shows that it has. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. Oxygen Magnesium Chloride Chemical Formula.
From studdy.in
4. Write the chemical formulae of the following Studdy Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. write the chemical formula for the compound that is formed when. Oxygen Magnesium Chloride Chemical Formula.
From www.youtube.com
How to Balance Mg(ClO3)2 = MgCl2 + O2 (Magnesium chlorate Oxygen Magnesium Chloride Chemical Formula magnesium chloride + oxygen produces magnesium chlorate. for example, magnesium oxide is made up of two elements, magnesium and oxygen. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has. Oxygen Magnesium Chloride Chemical Formula.
From www.youtube.com
Is MgCl2 (Magnesium chloride) Ionic or Covalent? YouTube Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium chloride is an ionic. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. magnesium chloride + oxygen produces magnesium chlorate.. Oxygen Magnesium Chloride Chemical Formula.
From manualfixageundeployed.z13.web.core.windows.net
Magnesium Atomic Structure Diagram Oxygen Magnesium Chloride Chemical Formula for example, magnesium oxide is made up of two elements, magnesium and oxygen. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom. Oxygen Magnesium Chloride Chemical Formula.
From www.slideshare.net
Chem matters ch6_ionic_bond Oxygen Magnesium Chloride Chemical Formula the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. This shows that it has. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. magnesium chloride + oxygen produces. Oxygen Magnesium Chloride Chemical Formula.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Oxygen Magnesium Chloride Chemical Formula for example, magnesium oxide is made up of two elements, magnesium and oxygen. magnesium chloride + oxygen produces magnesium chlorate. Butane, which has the chemical formula c₄h₁₀, has four carbon. what is the formula for barium chloride? when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. Oxygen Magnesium Chloride Chemical Formula.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Oxygen Magnesium Chloride Chemical Formula Butane, which has the chemical formula c₄h₁₀, has four carbon. for example, magnesium oxide is made up of two elements, magnesium and oxygen. This shows that it has. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. when an ionic compound is formed from. Oxygen Magnesium Chloride Chemical Formula.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. This shows that it has. for example, magnesium oxide is made up of two elements, magnesium and oxygen. what is the formula for barium chloride? Barium is an alkaline earth and always corms. Oxygen Magnesium Chloride Chemical Formula.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Magnesium chloride is an ionic. what is the formula for barium chloride? the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom. Oxygen Magnesium Chloride Chemical Formula.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Oxygen Magnesium Chloride Chemical Formula the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. magnesium chloride + oxygen produces magnesium chlorate. This shows that it has. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. Magnesium chloride. Oxygen Magnesium Chloride Chemical Formula.
From www.vrogue.co
1 Write The Molecular Formula For The Following By Cr vrogue.co Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. magnesium chloride + oxygen produces magnesium chlorate. Butane, which has the. Oxygen Magnesium Chloride Chemical Formula.
From gbu-presnenskij.ru
Draw The Lewis Structure Of MgCl2 (magnesium Chloride), 57 OFF Oxygen Magnesium Chloride Chemical Formula the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. magnesium chloride + oxygen produces magnesium chlorate. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. This shows that it has. for. Oxygen Magnesium Chloride Chemical Formula.
From www.slideshare.net
Bonding Basics Oxygen Magnesium Chloride Chemical Formula This shows that it has. for example, magnesium oxide is made up of two elements, magnesium and oxygen. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. Magnesium chloride is an ionic. write the chemical formula for the compound that is formed when each. Oxygen Magnesium Chloride Chemical Formula.
From mavink.com
Magnesium And Hcl Reaction Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Butane, which has the chemical formula c₄h₁₀, has four carbon. This shows that it has. magnesium chloride + oxygen produces magnesium chlorate. write the chemical formula for the compound that is formed when. Oxygen Magnesium Chloride Chemical Formula.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Oxygen Magnesium Chloride Chemical Formula Butane, which has the chemical formula c₄h₁₀, has four carbon. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. write the chemical formula for the compound that is formed when each of the following combinations of elements bond with one. when an ionic compound. Oxygen Magnesium Chloride Chemical Formula.
From www.numerade.com
SOLVED Write the chemical equation for the reaction of magnesium with Oxygen Magnesium Chloride Chemical Formula what is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every one atom of oxygen. Magnesium chloride is an ionic. when an ionic. Oxygen Magnesium Chloride Chemical Formula.
From www.youtube.com
How to Draw the MgCl2 Lewis Dot Structure. YouTube Oxygen Magnesium Chloride Chemical Formula magnesium chloride + oxygen produces magnesium chlorate. for example, magnesium oxide is made up of two elements, magnesium and oxygen. what is the formula for barium chloride? This shows that it has. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and. Magnesium chloride is an ionic.. Oxygen Magnesium Chloride Chemical Formula.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Oxygen Magnesium Chloride Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Butane, which has the chemical formula c₄h₁₀, has four carbon. Magnesium chloride is an ionic. the chemical formula for magnesium oxide, mgo, tells us that there is one atom of magnesium for every. Oxygen Magnesium Chloride Chemical Formula.