What Substances Dissolve In Water Chemistry . for example, when we breathe, the nitrogen in the air dissolves in our blood. Ionic compounds dissolve in water if the energy given. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. Learn more about how materials react to water in this bbc bitesize ks1 science. substances that dissolve in water to yield ions are called electrolytes. why do some material disappear in water? Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. water typically dissolves most ionic compounds and polar molecules. we can generally assume that salts dissociate into their ions when they dissolve in water. Nonpolar molecules, such as those found in grease.
from chem.libretexts.org
Nonpolar molecules, such as those found in grease. why do some material disappear in water? water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. we can generally assume that salts dissociate into their ions when they dissolve in water. substances that dissolve in water to yield ions are called electrolytes. Learn more about how materials react to water in this bbc bitesize ks1 science. for example, when we breathe, the nitrogen in the air dissolves in our blood.
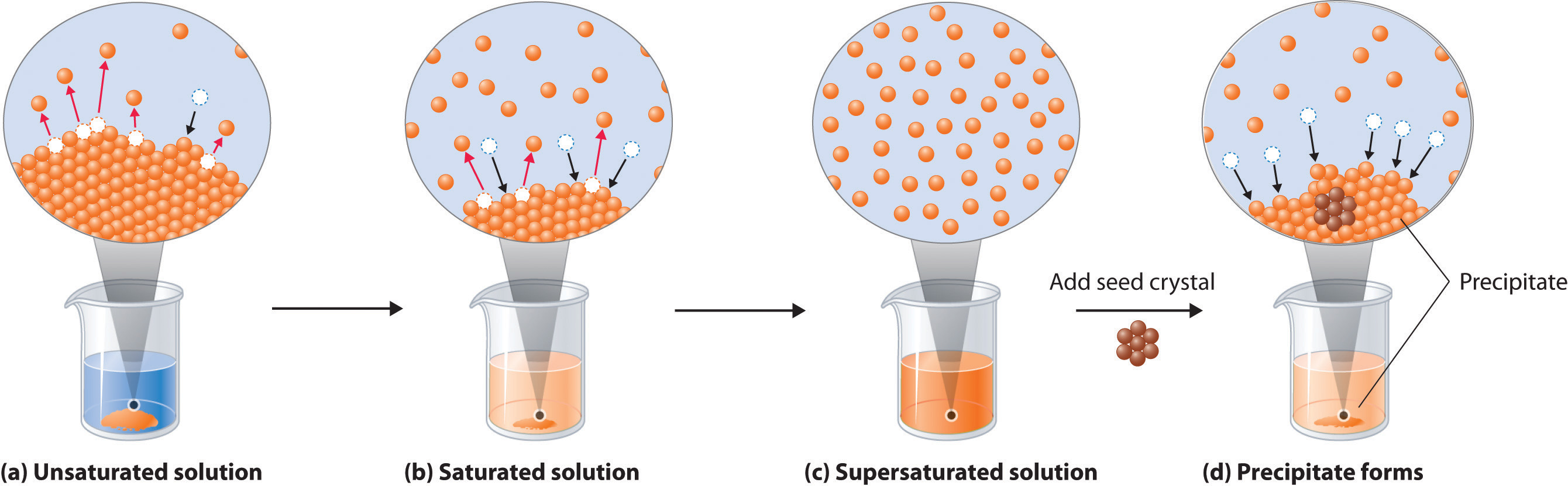
Solubility and Precipitation Chemistry LibreTexts
What Substances Dissolve In Water Chemistry Nonpolar molecules, such as those found in grease. substances that dissolve in water to yield ions are called electrolytes. Learn more about how materials react to water in this bbc bitesize ks1 science. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. Ionic compounds dissolve in water if the energy given. Nonelectrolytes are substances that do not. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. for example, when we breathe, the nitrogen in the air dissolves in our blood. water typically dissolves most ionic compounds and polar molecules. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. we can generally assume that salts dissociate into their ions when they dissolve in water. substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules, such as those found in grease. Nonelectrolytes are substances that do not produce ions. why do some material disappear in water?
From www.dreamstime.com
How Does Sodium Chloride NaCl Dissolve in Water Stock Vector What Substances Dissolve In Water Chemistry substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules, such as those found in grease. for example, when we breathe, the nitrogen in the air dissolves in our blood. Nonelectrolytes are substances that do not produce ions. water is a universal solvent because of its abundance and the fact that so many different. What Substances Dissolve In Water Chemistry.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Substances Dissolve In Water Chemistry Learn more about how materials react to water in this bbc bitesize ks1 science. Nonelectrolytes are substances that do not. why do some material disappear in water? Nonpolar molecules, such as those found in grease. substances that dissolve in water to yield ions are called electrolytes. we can generally assume that salts dissociate into their ions when. What Substances Dissolve In Water Chemistry.
From studydbtyler.z19.web.core.windows.net
Solubility And Concentration Worksheet What Substances Dissolve In Water Chemistry water typically dissolves most ionic compounds and polar molecules. Learn more about how materials react to water in this bbc bitesize ks1 science. substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules, such as those found in grease. Nonelectrolytes are substances that. What Substances Dissolve In Water Chemistry.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures What Substances Dissolve In Water Chemistry substances that dissolve in water to yield ions are called electrolytes. for example, when we breathe, the nitrogen in the air dissolves in our blood. Nonelectrolytes are substances that do not. Nonpolar molecules, such as those found in grease. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. water typically dissolves most ionic. What Substances Dissolve In Water Chemistry.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize What Substances Dissolve In Water Chemistry Nonpolar molecules, such as those found in grease. we can generally assume that salts dissociate into their ions when they dissolve in water. Nonelectrolytes are substances that do not produce ions. water typically dissolves most ionic compounds and polar molecules. why do some material disappear in water? substances that dissolve in water to yield ions are. What Substances Dissolve In Water Chemistry.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Substances Dissolve In Water Chemistry we can generally assume that salts dissociate into their ions when they dissolve in water. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Henry’s law gives. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID What Substances Dissolve In Water Chemistry Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. we can generally assume that salts dissociate into their ions when they dissolve in water. Nonelectrolytes are substances that do not produce ions. for example, when we breathe, the nitrogen in the air dissolves in our blood. Nonelectrolytes are. What Substances Dissolve In Water Chemistry.
From socratic.org
What is the chemical equation for HCl dissolving into water and What Substances Dissolve In Water Chemistry why do some material disappear in water? substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a.. What Substances Dissolve In Water Chemistry.
From littlebinsforlittlehands.com
Which Solids Dissolve In Water Chemistry for Kids What Substances Dissolve In Water Chemistry we can generally assume that salts dissociate into their ions when they dissolve in water. Nonpolar molecules, such as those found in grease. Ionic compounds dissolve in water if the energy given. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. for example, when we breathe, the nitrogen in the air dissolves in our. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Solution Properties PowerPoint Presentation, free download ID What Substances Dissolve In Water Chemistry water typically dissolves most ionic compounds and polar molecules. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules, such as those found in grease. substances that dissolve in water to yield ions are called electrolytes. why do some material. What Substances Dissolve In Water Chemistry.
From www.alamy.com
Solution science experiment. Solubility of salt and sand in water What Substances Dissolve In Water Chemistry water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Nonpolar molecules, such as those found in grease. Nonelectrolytes are substances that do not. Ionic compounds dissolve in water. What Substances Dissolve In Water Chemistry.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts What Substances Dissolve In Water Chemistry Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. substances that dissolve in water to yield ions are called electrolytes. water typically dissolves most ionic compounds and polar molecules. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve. What Substances Dissolve In Water Chemistry.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous What Substances Dissolve In Water Chemistry why do some material disappear in water? Learn more about how materials react to water in this bbc bitesize ks1 science. Nonpolar molecules, such as those found in grease. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. for example, when we breathe, the nitrogen in the air dissolves in our blood. Solute the. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 What Substances Dissolve In Water Chemistry Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Nonelectrolytes are substances that do not. substances that dissolve in water to yield ions are called electrolytes. Learn more about how materials react to. What Substances Dissolve In Water Chemistry.
From www.twinkl.kr
What is Dissolving? Answered Twinkl Teaching Wiki What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not produce ions. Ionic compounds dissolve in water if the energy given. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. substances that dissolve in water to yield ions are called electrolytes. Learn more about how materials react to water in this bbc bitesize. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Basic Principles of Chemistry PowerPoint Presentation, free What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. water is a universal solvent because of its abundance and the fact that so. What Substances Dissolve In Water Chemistry.
From www.chemicals.co.uk
What is Solubility? The Chemistry Blog What Substances Dissolve In Water Chemistry water typically dissolves most ionic compounds and polar molecules. Learn more about how materials react to water in this bbc bitesize ks1 science. Nonelectrolytes are substances that do not produce ions. why do some material disappear in water? Nonelectrolytes are substances that do not. Ionic compounds dissolve in water if the energy given. we can generally assume. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not. substances that dissolve in water to yield ions are called electrolytes. we can generally assume that salts dissociate into their ions when they dissolve in water. substances that dissolve in water to yield ions are called electrolytes. water is a universal solvent because of its abundance and the fact that. What Substances Dissolve In Water Chemistry.
From www.slideshare.net
Water and solutions What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not. Ionic compounds dissolve in water if the energy given. we can generally assume that salts dissociate into their ions when they dissolve in water. water typically dissolves most ionic compounds and polar molecules. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve. What Substances Dissolve In Water Chemistry.
From www.youtube.com
Class 6 Science Separation of Substances Solubility in Water What Substances Dissolve In Water Chemistry Ionic compounds dissolve in water if the energy given. why do some material disappear in water? we can generally assume that salts dissociate into their ions when they dissolve in water. Learn more about how materials react to water in this bbc bitesize ks1 science. Nonpolar molecules, such as those found in grease. water typically dissolves most. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Lec.1 Chemistry Of Water PowerPoint Presentation, free download What Substances Dissolve In Water Chemistry substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. Ionic compounds dissolve in water if the energy given. Nonpolar molecules, such as those found in grease. substances that dissolve in water to yield ions are called electrolytes. water is a universal solvent because of its abundance and. What Substances Dissolve In Water Chemistry.
From webmis.highland.cc.il.us
Aqueous Solutions What Substances Dissolve In Water Chemistry Nonpolar molecules, such as those found in grease. water typically dissolves most ionic compounds and polar molecules. Ionic compounds dissolve in water if the energy given. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Nonelectrolytes are substances that do not. substances that dissolve in. What Substances Dissolve In Water Chemistry.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not. we can generally assume that salts dissociate into their ions when they dissolve in water. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. why do some material disappear in water? Henry’s law gives us the power to predict,. What Substances Dissolve In Water Chemistry.
From userdatarheumatics.z21.web.core.windows.net
Diagram Of Salt Dissolving In Water What Substances Dissolve In Water Chemistry why do some material disappear in water? Nonpolar molecules, such as those found in grease. for example, when we breathe, the nitrogen in the air dissolves in our blood. Nonelectrolytes are substances that do not. we can generally assume that salts dissociate into their ions when they dissolve in water. Learn more about how materials react to. What Substances Dissolve In Water Chemistry.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not. Ionic compounds dissolve in water if the energy given. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid). What Substances Dissolve In Water Chemistry.
From saylordotorg.github.io
Aqueous Solutions What Substances Dissolve In Water Chemistry substances that dissolve in water to yield ions are called electrolytes. water typically dissolves most ionic compounds and polar molecules. we can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given. substances that dissolve in water to yield ions are called electrolytes. . What Substances Dissolve In Water Chemistry.
From www.twinkl.co.uk
What is Dissolving? Answered Twinkl Teaching Wiki What Substances Dissolve In Water Chemistry Ionic compounds dissolve in water if the energy given. water typically dissolves most ionic compounds and polar molecules. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. Learn more about how materials react to water in. What Substances Dissolve In Water Chemistry.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not. substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules, such as those found in grease. substances that dissolve in water to yield ions are called electrolytes. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Learn more about. What Substances Dissolve In Water Chemistry.
From www.breakingatom.com
Solubility of Elements and Compounds What Substances Dissolve In Water Chemistry Nonelectrolytes are substances that do not produce ions. Ionic compounds dissolve in water if the energy given. we can generally assume that salts dissociate into their ions when they dissolve in water. substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules, such as those found in grease. Nonelectrolytes are substances that do not. . What Substances Dissolve In Water Chemistry.
From sciencenotes.org
Why Is Water Called the Universal Solvent? What Substances Dissolve In Water Chemistry we can generally assume that salts dissociate into their ions when they dissolve in water. for example, when we breathe, the nitrogen in the air dissolves in our blood. Nonpolar molecules, such as those found in grease. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. substances that dissolve in water to yield. What Substances Dissolve In Water Chemistry.
From studypositivity.z21.web.core.windows.net
How Does Water Dissolve Substances What Substances Dissolve In Water Chemistry substances that dissolve in water to yield ions are called electrolytes. Learn more about how materials react to water in this bbc bitesize ks1 science. we can generally assume that salts dissociate into their ions when they dissolve in water. water typically dissolves most ionic compounds and polar molecules. Ionic compounds dissolve in water if the energy. What Substances Dissolve In Water Chemistry.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes What Substances Dissolve In Water Chemistry we can generally assume that salts dissociate into their ions when they dissolve in water. Learn more about how materials react to water in this bbc bitesize ks1 science. Ionic compounds dissolve in water if the energy given. why do some material disappear in water? Nonelectrolytes are substances that do not. Solute the solid (or occasionally a gas). What Substances Dissolve In Water Chemistry.
From courses.lumenlearning.com
The Dissolution Process Chemistry What Substances Dissolve In Water Chemistry Ionic compounds dissolve in water if the energy given. Nonelectrolytes are substances that do not produce ions. Henry’s law gives us the power to predict, prevent, and treat “the bends”—a. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. water is a universal solvent because of its abundance and. What Substances Dissolve In Water Chemistry.
From www.ck12.org
Solubility CK12 Foundation What Substances Dissolve In Water Chemistry Ionic compounds dissolve in water if the energy given. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Nonelectrolytes are substances that do not. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. we can generally. What Substances Dissolve In Water Chemistry.
From www.thoughtco.com
Dissolve Definition in Chemistry What Substances Dissolve In Water Chemistry Ionic compounds dissolve in water if the energy given. water is a universal solvent because of its abundance and the fact that so many different compounds dissolve in it (sect. Nonelectrolytes are substances that do not. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. Henry’s law gives. What Substances Dissolve In Water Chemistry.