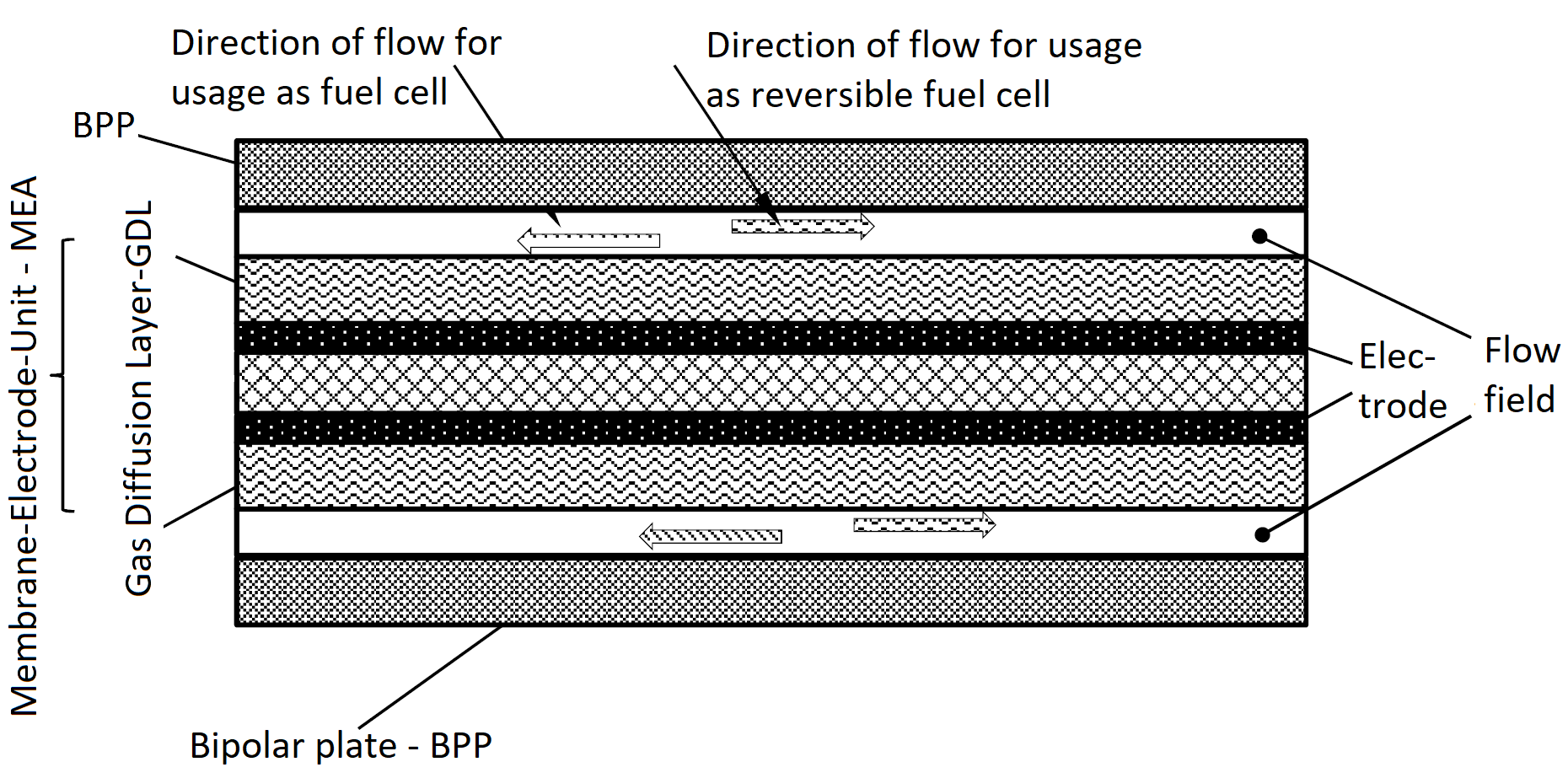
Difference Between Fuel Cells And Electrolyzers . Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Understand the relationship between gibbs free energy and electrochemical cell potential. Electrolysis is the process of using electricity to split water into hydrogen and. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. However, one difference from fuel cells is. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells.
from techoffers.tu-berlin.de
A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: However, one difference from fuel cells is. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Electrolysis is the process of using electricity to split water into hydrogen and. Understand the relationship between gibbs free energy and electrochemical cell potential.
Catalyst material for Fuel Cells, Electrolyzers, and a Manufacturing
Difference Between Fuel Cells And Electrolyzers A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Understand the relationship between gibbs free energy and electrochemical cell potential. Electrolysis is the process of using electricity to split water into hydrogen and. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. However, one difference from fuel cells is. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range.
From farrahqrosanna.pages.dev
Difference Between Fuel Cell And Battery Electric Vehicles Meaning Difference Between Fuel Cells And Electrolyzers However, one difference from fuel cells is. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen. Difference Between Fuel Cells And Electrolyzers.
From techoffers.tu-berlin.de
Catalyst material for Fuel Cells, Electrolyzers, and a Manufacturing Difference Between Fuel Cells And Electrolyzers Electrolysis is the process of using electricity to split water into hydrogen and. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: Understand the relationship between gibbs free energy and electrochemical cell potential. However, one difference from fuel cells is. There are many ways to. Difference Between Fuel Cells And Electrolyzers.
From www.miragenews.com
Using excess heat to improve electrolyzers and fuel cells Mirage News Difference Between Fuel Cells And Electrolyzers There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time. Difference Between Fuel Cells And Electrolyzers.
From www.youtube.com
3 Benefits of Wire Mesh for Electrolyzers & Fuel Cells Gerard Daniel Difference Between Fuel Cells And Electrolyzers Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Understand the relationship between gibbs free energy and electrochemical cell potential. Electrolysis is the process of using electricity to split water into hydrogen and. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two. Difference Between Fuel Cells And Electrolyzers.
From www.mdpi.com
Electrochem Free FullText NiFeOx and NiFeCoOx Catalysts for Anion Difference Between Fuel Cells And Electrolyzers A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. However, one difference from fuel cells is. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells. Difference Between Fuel Cells And Electrolyzers.
From www.mathworks.com
Simulink for Fuel Cells and Electrolyzers MATLAB & Simulink Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. However, one difference from fuel cells is. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen and. A significant advantage of pem electrolyzers and fuel. Difference Between Fuel Cells And Electrolyzers.
From convergecfd.com
Simulating Fuel Cells and Electrolyzers With CONVERGE CONVERGE CFD Difference Between Fuel Cells And Electrolyzers Electrolysis is the process of using electricity to split water into hydrogen and. Understand the relationship between gibbs free energy and electrochemical cell potential. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. There are many ways to build and configure an electrolyzer, and different electrolytes can be used. Difference Between Fuel Cells And Electrolyzers.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5431275 Difference Between Fuel Cells And Electrolyzers Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: However, one difference from fuel cells is. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Understand the relationship between gibbs free energy and. Difference Between Fuel Cells And Electrolyzers.
From powerup-tech.com
The ABC of Fuel Cells PowerUP Energy Technologies Difference Between Fuel Cells And Electrolyzers Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: Electrolysis is the process of using electricity to split water into hydrogen and. However, one difference. Difference Between Fuel Cells And Electrolyzers.
From www.ourenergypolicy.org
Water Electrolyzers and Fuel Cells Supply Chain Deep Dive Assessment Difference Between Fuel Cells And Electrolyzers A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen and. Green hydrogen energy (ghe) storage, using. Difference Between Fuel Cells And Electrolyzers.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Difference Between Fuel Cells And Electrolyzers There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen and. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Polymer electrolyte membrane (pem) fuel cells. Difference Between Fuel Cells And Electrolyzers.
From www.equities.com
Will the Fuel Cells Vehicles Save Platinum and Palladium? Equities News Difference Between Fuel Cells And Electrolyzers A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: However, one difference from fuel cells is. Electrolysis is the process of using electricity to split. Difference Between Fuel Cells And Electrolyzers.
From yocharge.com
Difference between Battery and Fuel Cells YoCharge Difference Between Fuel Cells And Electrolyzers However, one difference from fuel cells is. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: Electrolysis is the process of using electricity to split water into hydrogen and. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as. Difference Between Fuel Cells And Electrolyzers.
From www.slideserve.com
PPT Fuel Cell Technology PowerPoint Presentation, free download ID Difference Between Fuel Cells And Electrolyzers Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: However, one difference from fuel cells is. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. There are many ways to build and configure an. Difference Between Fuel Cells And Electrolyzers.
From exyamfqge.blob.core.windows.net
Advantages Of Hydrogen Fuel Cells Gcse Chemistry at Clinton Earl blog Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Electrolysis is the process of using electricity to split water into hydrogen and. However, one difference from fuel cells is. There are many ways to build and configure an. Difference Between Fuel Cells And Electrolyzers.
From www.researchgate.net
Schematic of polymer electrolyte membrane (PEM) methanol electrolyzer Difference Between Fuel Cells And Electrolyzers Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel. Difference Between Fuel Cells And Electrolyzers.
From cicenergigune.com
Fuel cells, decoupled electrolyzers... The key hydrogen concepts that Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. However, one difference from fuel cells is. Electrolysis is the process of using electricity to split water into hydrogen and. Polymer electrolyte membrane (pem) fuel cells or pemfcs and. Difference Between Fuel Cells And Electrolyzers.
From energy.gov
Hydrogen Production Electrolysis Department of Energy Difference Between Fuel Cells And Electrolyzers Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Understand the relationship between gibbs free energy and electrochemical cell potential. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Polymer electrolyte membrane (pem) fuel cells or pemfcs. Difference Between Fuel Cells And Electrolyzers.
From www.differencebetween.com
Difference Between Fuel Cell and Battery Compare the Difference Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: Electrolysis is the process of using electricity to split water into hydrogen and. A significant advantage of pem electrolyzers and fuel cells is their extremely. Difference Between Fuel Cells And Electrolyzers.
From www.researchgate.net
Configurations for water electrolysis (a) proton exchange membrane Difference Between Fuel Cells And Electrolyzers However, one difference from fuel cells is. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: Electrolysis is the process of using electricity to split. Difference Between Fuel Cells And Electrolyzers.
From www.researchgate.net
Principle of operation of proton exchange membrane (PEM) electrolyzers Difference Between Fuel Cells And Electrolyzers Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Electrolysis is the process of using electricity to split water into hydrogen and. However, one difference. Difference Between Fuel Cells And Electrolyzers.
From www.biologic.net
What are PEM Fuel Cells and Electrolyzers? BioLogic Learning Center Difference Between Fuel Cells And Electrolyzers However, one difference from fuel cells is. Electrolysis is the process of using electricity to split water into hydrogen and. Understand the relationship between gibbs free energy and electrochemical cell potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: There are many ways to. Difference Between Fuel Cells And Electrolyzers.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Difference Between Fuel Cells And Electrolyzers Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: Understand the relationship between gibbs free energy and electrochemical cell potential. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. However, one difference from fuel. Difference Between Fuel Cells And Electrolyzers.
From www.researchgate.net
Schematic representation of AEM fuel cells (top) and electrolyzers Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. Electrolysis is the process of using electricity to split water into hydrogen and. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. However, one difference from fuel cells is. Green hydrogen energy (ghe) storage, using electrolyzers (el). Difference Between Fuel Cells And Electrolyzers.
From www.biologic.net
What are PEM Fuel Cells and Electrolyzers? BioLogic Learning Center Difference Between Fuel Cells And Electrolyzers There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical. Difference Between Fuel Cells And Electrolyzers.
From www.caplinq.com
Carbon papers for Fuel Cells & Electrolyzers CAPLINQ BLOG Difference Between Fuel Cells And Electrolyzers Electrolysis is the process of using electricity to split water into hydrogen and. However, one difference from fuel cells is. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over. Difference Between Fuel Cells And Electrolyzers.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Difference Between Fuel Cells And Electrolyzers However, one difference from fuel cells is. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices having a similar structure: A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Green hydrogen energy (ghe) storage, using electrolyzers (el) and. Difference Between Fuel Cells And Electrolyzers.
From www.theecoexperts.co.uk
Fuel Cells vs Batteries What’s the Difference? Eco Experts Difference Between Fuel Cells And Electrolyzers Electrolysis is the process of using electricity to split water into hydrogen and. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Understand the relationship between gibbs free. Difference Between Fuel Cells And Electrolyzers.
From www.outokumpu.com
Stainless steel provides a solid future for fuel cells and Difference Between Fuel Cells And Electrolyzers Electrolysis is the process of using electricity to split water into hydrogen and. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. There are many ways to build and. Difference Between Fuel Cells And Electrolyzers.
From www.slideserve.com
PPT Fuel Cells and Rechargeable Batteries C5 PowerPoint Presentation Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen and. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are. Difference Between Fuel Cells And Electrolyzers.
From www.youtube.com
Comparison of Fuel Cells YouTube Difference Between Fuel Cells And Electrolyzers However, one difference from fuel cells is. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells. Difference Between Fuel Cells And Electrolyzers.
From kladutthv.blob.core.windows.net
Fuel Cell Electrolysis at Howard Rooks blog Difference Between Fuel Cells And Electrolyzers A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. Polymer electrolyte membrane (pem) fuel cells or pemfcs and pem electrolysis cells or pemecs are two closely related electrochemical devices. Difference Between Fuel Cells And Electrolyzers.
From www.victoriana.com
Matratze Lavendel Feindseligkeit difference between fuel cell and Difference Between Fuel Cells And Electrolyzers A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen and. Green hydrogen energy (ghe) storage, using. Difference Between Fuel Cells And Electrolyzers.
From www.researchgate.net
(PDF) Hydrocarbon Ionomeric Binders for Fuel Cells and Electrolyzers Difference Between Fuel Cells And Electrolyzers There are many ways to build and configure an electrolyzer, and different electrolytes can be used just like in fuel cells. Electrolysis is the process of using electricity to split water into hydrogen and. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Polymer electrolyte membrane (pem) fuel cells. Difference Between Fuel Cells And Electrolyzers.
From www.biologic.net
What are PEM Fuel Cells and Electrolyzers? BioLogic Learning Center Difference Between Fuel Cells And Electrolyzers Understand the relationship between gibbs free energy and electrochemical cell potential. Green hydrogen energy (ghe) storage, using electrolyzers (el) and fuel cells (fc), has been identified as one of the potential. However, one difference from fuel cells is. A significant advantage of pem electrolyzers and fuel cells is their extremely rapid response time over a large load range. Electrolysis is. Difference Between Fuel Cells And Electrolyzers.