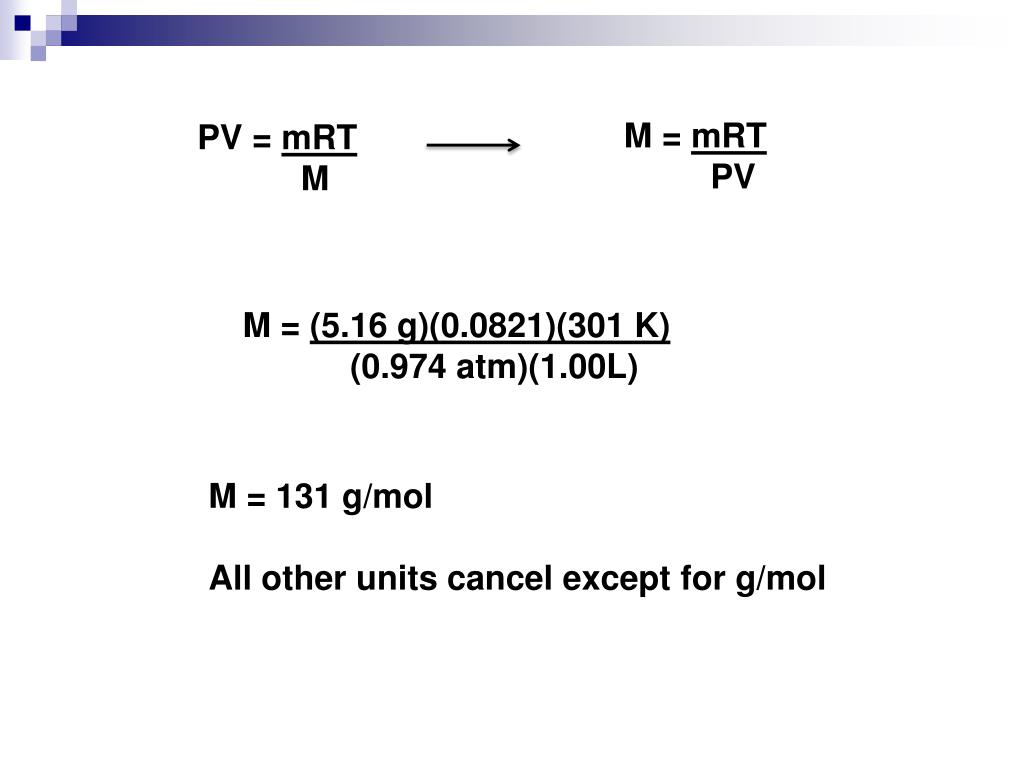
Pi = Mrt Equation . I is the van’t hoff factor. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. C is the molar concentration of the. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: M is the molar concentration of dissolved species (units of. Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be calculated with the help of the following formula: Osmotic pressure obeys a law that resembles the ideal gas equation: Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. Osmotic pressure obeys a law that resembles. Π is not equal to 3.14159 in this situation. Osmotic pressure is expressed by the formula:
from www.slideserve.com
The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: M is the molar concentration of dissolved species (units of. Osmotic pressure obeys a law that resembles the ideal gas equation: Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. I is the van’t hoff factor. C is the molar concentration of the. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is.
PPT Gases and Gas Laws PowerPoint Presentation, free download ID
Pi = Mrt Equation I is the van’t hoff factor. Osmotic pressure can be calculated with the help of the following formula: Osmotic pressure obeys a law that resembles the ideal gas equation: Where, π is the osmotic pressure. C is the molar concentration of the. I is the van’t hoff factor. Osmotic pressure is expressed by the formula: Osmotic pressure obeys a law that resembles. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Π is not equal to 3.14159 in this situation. Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. M is the molar concentration of dissolved species (units of. We calculate the osmotic pressure, (pi), using the following equation:
From www.youtube.com
How to Solve for the MRTS Five Examples YouTube Pi = Mrt Equation The osmotic pressure of a solution depends on the concentration of dissolved solute particles. C is the molar concentration of the. Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. Osmotic pressure is expressed by the formula: We calculate the osmotic pressure, (pi), using the following equation: Osmotic pressure obeys a law that. Pi = Mrt Equation.
From www.youtube.com
How to find CF and PI of a linear differential equation Some Pi = Mrt Equation We calculate the osmotic pressure, (pi), using the following equation: I is the van’t hoff factor. Where, π is the osmotic pressure. C is the molar concentration of the. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin. Pi = Mrt Equation.
From www.slideserve.com
PPT SEMINAR ON PowerPoint Pi = Mrt Equation Osmotic pressure obeys a law that resembles the ideal gas equation: Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: Osmotic pressure obeys a law that resembles. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is. Pi = Mrt Equation.
From theconversation.com
Pi and its part in the most beautiful formula in mathematics Pi = Mrt Equation M is the molar concentration of dissolved species (units of. Π is not equal to 3.14159 in this situation. Osmotic pressure is expressed by the formula: C is the molar concentration of the. Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: Osmotic pressure obeys a law that resembles. The osmotic pressure of. Pi = Mrt Equation.
From quizretentives.z13.web.core.windows.net
How To Know The Vant Hoff Factor Pi = Mrt Equation Osmotic pressure obeys a law that resembles the ideal gas equation: Osmotic pressure obeys a law that resembles. Osmotic pressure is expressed by the formula: Where, π is the osmotic pressure. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. The osmotic pressure of a solution depends on the. Pi = Mrt Equation.
From www.toppr.com
Pi Formula Concept, Interesting Facts, Solved Examples Pi = Mrt Equation Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. I is the van’t hoff factor. M is the molar concentration of dissolved species (units of. Π is not equal to 3.14159 in this situation. C is the molar concentration of the. Osmotic pressure can be calculated with the help. Pi = Mrt Equation.
From drrajivdesaimd.com
Mathematics of Pi Dr Rajiv Desai Pi = Mrt Equation I is the van’t hoff factor. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. C is the molar concentration of the. Osmotic pressure obeys a law that resembles. Osmotic pressure is expressed by the formula: Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: M is the. Pi = Mrt Equation.
From www.coursehero.com
[Solved] detailed steps. 2.9 (A) Derive the entropy equation for a Pi = Mrt Equation Osmotic pressure obeys a law that resembles. Π is not equal to 3.14159 in this situation. Osmotic pressure obeys a law that resembles the ideal gas equation: M is the molar concentration of dissolved species (units of. Osmotic pressure can be calculated with the help of the following formula: C is the molar concentration of the. Π stands for the. Pi = Mrt Equation.
From www.coursehero.com
[Solved] Consider the ideal gas equation of state PV = mRT = nRT where Pi = Mrt Equation C is the molar concentration of the. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure obeys a law that resembles. Π is not equal to 3.14159 in this situation. Osmotic pressure can be calculated with the help of the following formula: Osmotic pressure obeys a law that resembles the ideal gas equation:. Pi = Mrt Equation.
From medium.com
The Beauties Hidden in Pi(π). It was finally the weekend! After my Pi = Mrt Equation Osmotic pressure is expressed by the formula: Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. Osmotic pressure obeys a law that resembles. We calculate the osmotic pressure, (pi), using the following equation: M is the molar concentration of dissolved species (units of. Where, π is the osmotic pressure. The osmotic pressure of. Pi = Mrt Equation.
From owlcation.com
How to Calculate the Area of Circle in Terms of Pi (π) Owlcation Pi = Mrt Equation Osmotic pressure can be calculated with the help of the following formula: M is the molar concentration of dissolved species (units of. Osmotic pressure obeys a law that resembles the ideal gas equation: The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Π = imrt (note how it resembles the pv = nrt form of. Pi = Mrt Equation.
From www.youtube.com
Osmotic Pressure π = MRT YouTube Pi = Mrt Equation Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. Π = imrt (note how it resembles the pv = nrt form of the. Pi = Mrt Equation.
From fermancebo.com
Direct formula of Pi Pythagoras composition Pi = Mrt Equation Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres.. Pi = Mrt Equation.
From www.chegg.com
Solved PMRT 9 1. Which one of the following equations Pi = Mrt Equation Osmotic pressure is expressed by the formula: Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. We calculate the osmotic pressure, (pi), using the following equation: M is the molar concentration of dissolved species (units of. Π stands for the osmotic pressure and is usually expressed in the pressure. Pi = Mrt Equation.
From www.coursehero.com
[Solved] . Consider the ideal gas equation of state PV = mRT = ART R Pi = Mrt Equation The osmotic pressure of a solution depends on the concentration of dissolved solute particles. C is the molar concentration of the. Osmotic pressure obeys a law that resembles. We calculate the osmotic pressure, (pi), using the following equation: I is the van’t hoff factor. M is the molar concentration of dissolved species (units of. Where, π is the osmotic pressure.. Pi = Mrt Equation.
From aboutradiation.blogspot.com
Imrt Osmotic Pressure All About Radiation Pi = Mrt Equation Π is not equal to 3.14159 in this situation. C is the molar concentration of the. Osmotic pressure obeys a law that resembles. We calculate the osmotic pressure, (pi), using the following equation: Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. Π stands for the osmotic pressure and. Pi = Mrt Equation.
From www.numerade.com
SOLVED Osmotic pressure (π) can be calculated using the equation π=M R Pi = Mrt Equation The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure obeys a law that resembles the ideal gas equation: Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. Π is not equal to. Pi = Mrt Equation.
From www.ck12.org
Electrolytes and Colligative Properties CK12 Foundation Pi = Mrt Equation M is the molar concentration of dissolved species (units of. Π is not equal to 3.14159 in this situation. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. C is the molar concentration of the. Osmotic pressure is expressed. Pi = Mrt Equation.
From www.coursehero.com
[Solved] . Consider the ideal gas equation of state PV = mRT = ART R Pi = Mrt Equation I is the van’t hoff factor. M is the molar concentration of dissolved species (units of. Osmotic pressure obeys a law that resembles. Osmotic pressure is expressed by the formula: We calculate the osmotic pressure, (pi), using the following equation: Where, π is the osmotic pressure. Find the osmotic pressure, π, using the formula π = mrt, where t is. Pi = Mrt Equation.
From www.youtube.com
MRTS with calculus YouTube Pi = Mrt Equation M is the molar concentration of dissolved species (units of. Osmotic pressure is expressed by the formula: Osmotic pressure obeys a law that resembles the ideal gas equation: Π is not equal to 3.14159 in this situation. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. The osmotic pressure. Pi = Mrt Equation.
From mindyourdecisions.com
The Wallis Product Formula For Pi And Its Proof Mind Your Decisions Pi = Mrt Equation Osmotic pressure obeys a law that resembles the ideal gas equation: Π is not equal to 3.14159 in this situation. Osmotic pressure obeys a law that resembles. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. The osmotic pressure. Pi = Mrt Equation.
From www.ck12.org
Electrolytes and Colligative Properties CK12 Foundation Pi = Mrt Equation The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. I is the van’t hoff factor. C is the molar concentration of the. Where, π is. Pi = Mrt Equation.
From www.gizmodo.com.au
Pi, And Its Part In The Most Beautiful Formula In Maths Gizmodo Australia Pi = Mrt Equation Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. We calculate the osmotic pressure, (pi), using the following equation: Osmotic pressure obeys a law that resembles. Osmotic pressure can be calculated with the help of the following formula: The osmotic pressure of a solution depends on the concentration of. Pi = Mrt Equation.
From www.slideserve.com
PPT Gases and Gas Laws PowerPoint Presentation, free download ID Pi = Mrt Equation Osmotic pressure is expressed by the formula: Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure obeys a law that resembles. Find the osmotic pressure, π, using the formula π = mrt, where t is on the. Pi = Mrt Equation.
From www.pinterest.com
An artist painted pi to 2,500 decimal places to make math more Pi = Mrt Equation M is the molar concentration of dissolved species (units of. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. Π stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. Osmotic pressure obeys a law that resembles the ideal gas equation: C is the. Pi = Mrt Equation.
From studylib.net
Ideal Gas Law P V = n R T Pi = Mrt Equation The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure is expressed by the formula: Π is not equal to 3.14159 in this situation. Where, π is the osmotic pressure. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. M is the molar. Pi = Mrt Equation.
From www.medschoolcoach.com
Zwitterions and Isoelectric Point MCAT Biochemistry MedSchoolCoach Pi = Mrt Equation Osmotic pressure can be calculated with the help of the following formula: Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units. Where, π is the osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved. Pi = Mrt Equation.
From www.slideserve.com
PPT Mole Calculations PowerPoint Presentation ID902714 Pi = Mrt Equation Osmotic pressure can be calculated with the help of the following formula: Osmotic pressure obeys a law that resembles the ideal gas equation: Osmotic pressure is expressed by the formula: Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is expressed in appropriate units.. Pi = Mrt Equation.
From www.youtube.com
10. CF & PI Problem1 DIFFERENTIAL EQUATIONS OF HIGHER ORDER YouTube Pi = Mrt Equation Osmotic pressure is expressed by the formula: The osmotic pressure of a solution depends on the concentration of dissolved solute particles. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure obeys a law that resembles the ideal gas equation: I is the van’t hoff factor. Π is not equal to 3.14159 in this. Pi = Mrt Equation.
From www.toppr.com
Using the equation of state pV = nRT ; show that at a given temperature Pi = Mrt Equation The osmotic pressure of a solution depends on the concentration of dissolved solute particles. I is the van’t hoff factor. Osmotic pressure obeys a law that resembles the ideal gas equation: Where, π is the osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be calculated with the help of. Pi = Mrt Equation.
From www.pinterest.com
Vant Hoff formula For calculation of Osmotic pressure ( Note Pi = Mrt Equation Osmotic pressure obeys a law that resembles the ideal gas equation: The osmotic pressure of a solution depends on the concentration of dissolved solute particles. We calculate the osmotic pressure, (pi), using the following equation: Osmotic pressure can be calculated with the help of the following formula: Find the osmotic pressure, π, using the formula π = mrt, where t. Pi = Mrt Equation.
From www.chegg.com
Solved Use pi = MRT and R = 0.0821 L*atm/mol*K to solve the Pi = Mrt Equation Osmotic pressure is expressed by the formula: C is the molar concentration of the. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. M is the molar concentration of dissolved species (units of. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Where, π. Pi = Mrt Equation.
From ar.inspiredpencil.com
Osmotic Pressure Formula Pi = Mrt Equation C is the molar concentration of the. Osmotic pressure is expressed by the formula: Π is not equal to 3.14159 in this situation. Where, π is the osmotic pressure. We calculate the osmotic pressure, (pi), using the following equation: The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Find the osmotic pressure, π, using the. Pi = Mrt Equation.
From www.chm.bris.ac.uk
Mass Spectrometry Facility FTICRMS Pi = Mrt Equation Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. Osmotic pressure obeys a law that resembles the ideal gas equation: Osmotic pressure obeys a law that resembles. We calculate the osmotic pressure, (pi), using the following equation: The osmotic pressure of a solution depends on the concentration of dissolved. Pi = Mrt Equation.
From www.liambalton.com
Make Pi Equation About Liam Pi = Mrt Equation Osmotic pressure is expressed by the formula: C is the molar concentration of the. Π = imrt (note how it resembles the pv = nrt form of the ideal gas law) where π is. Find the osmotic pressure, π, using the formula π = mrt, where t is on the kelvin scale (310 k) and the value of r is. Pi = Mrt Equation.