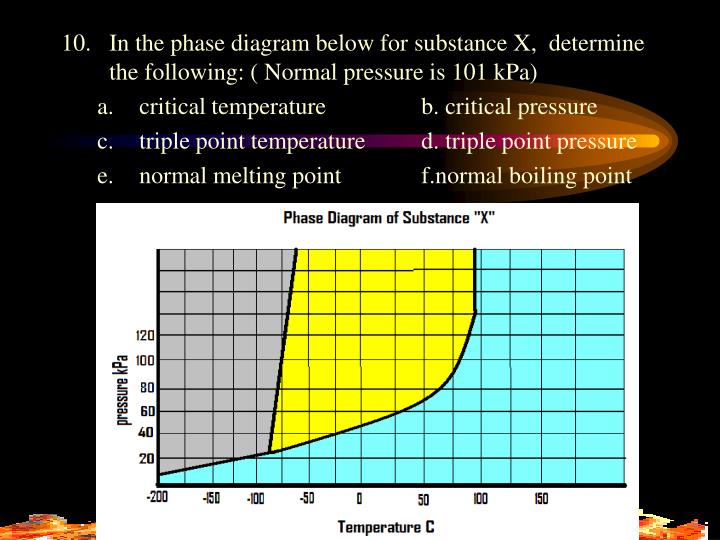
Phase Diagram Labeled Boiling Point . Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. In a phase diagram, pressure is plotted against temperature. The blue line is the boiling point. The solid green line shows the melting point of most liquids. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The phase diagram below is for methane, ch 4. Notice that the boiling temperature changes a lot with a change in pressure. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Note, the axes are not drawn to.
from mavink.com
These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Note, the axes are not drawn to. In a phase diagram, pressure is plotted against temperature. Solid, liquid, gas, and a supercritical fluid. The phase diagram below is for methane, ch 4. Notice that the boiling temperature changes a lot with a change in pressure. The blue line is the boiling point. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. To be able to identify the triple point, the critical point, and four regions:
Phase Diagram Boiling Point
Phase Diagram Labeled Boiling Point The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Notice that the boiling temperature changes a lot with a change in pressure. The phase diagram below is for methane, ch 4. Note, the axes are not drawn to. The blue line is the boiling point. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The solid green line shows the melting point of most liquids. Solid, liquid, gas, and a supercritical fluid. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. To be able to identify the triple point, the critical point, and four regions: In a phase diagram, pressure is plotted against temperature. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure.
From schematicgonorejomg2zmq.z13.web.core.windows.net
Phase Diagram Labeled Boiling Point Phase Diagram Labeled Boiling Point The solid green line shows the melting point of most liquids. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. Note, the axes are not drawn to. The blue line is the boiling point. The point where liquid become stable is called the triple point, where all three phases. Phase Diagram Labeled Boiling Point.
From glossary.periodni.com
Phase diagram Chemistry Dictionary & Glossary Phase Diagram Labeled Boiling Point The phase diagram below is for methane, ch 4. Note, the axes are not drawn to. The solid green line shows the melting point of most liquids. Solid, liquid, gas, and a supercritical fluid. In a phase diagram, pressure is plotted against temperature. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also. Phase Diagram Labeled Boiling Point.
From imfsrule.weebly.com
Phase Diagrams Intermolecular Forces Phase Diagram Labeled Boiling Point The phase diagram below is for methane, ch 4. The blue line is the boiling point. In a phase diagram, pressure is plotted against temperature. Solid, liquid, gas, and a supercritical fluid. The solid green line shows the melting point of most liquids. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also. Phase Diagram Labeled Boiling Point.
From jumpstarterdiscount.blogspot.com
Normal Boiling Point On Phase Diagram Wiring Diagram Phase Diagram Labeled Boiling Point The solid green line shows the melting point of most liquids. To be able to identify the triple point, the critical point, and four regions: The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. These diagrams indicate the physical states that exist under specific conditions of. Phase Diagram Labeled Boiling Point.
From guidedatadarren.z21.web.core.windows.net
How To Find Boiling Point On Phase Diagram Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The phase diagram below is for methane, ch 4. Note, the axes are not drawn to. To be able to identify the triple point, the critical point, and four regions: Notice that the boiling temperature changes a lot with a. Phase Diagram Labeled Boiling Point.
From guidemanualeruptivity.z14.web.core.windows.net
Phase Diagram Labeled Boiling Point Phase Diagram Labeled Boiling Point The blue line is the boiling point. Notice that the boiling temperature changes a lot with a change in pressure. Note, the axes are not drawn to. In a phase diagram, pressure is plotted against temperature. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The point where liquid. Phase Diagram Labeled Boiling Point.
From socratic.org
What is the relation between critical temperature and boiling point or Phase Diagram Labeled Boiling Point The solid green line shows the melting point of most liquids. The blue line is the boiling point. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. In a phase diagram, pressure is plotted against temperature. Notice that the boiling temperature changes a lot with a change in pressure.. Phase Diagram Labeled Boiling Point.
From wirepartpate.z13.web.core.windows.net
How To Read A Phase Change Diagram Phase Diagram Labeled Boiling Point Note, the axes are not drawn to. To be able to identify the triple point, the critical point, and four regions: The solid green line shows the melting point of most liquids. The blue line is the boiling point. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all. Phase Diagram Labeled Boiling Point.
From schematicfixexternal.z22.web.core.windows.net
Normal Boiling Point On Phase Diagram Phase Diagram Labeled Boiling Point In a phase diagram, pressure is plotted against temperature. The solid green line shows the melting point of most liquids. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure.. Phase Diagram Labeled Boiling Point.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Phase Diagram Labeled Boiling Point Note, the axes are not drawn to. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The point where liquid become stable is called the triple point, where all. Phase Diagram Labeled Boiling Point.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Phase Diagram Labeled Boiling Point The solid green line shows the melting point of most liquids. Solid, liquid, gas, and a supercritical fluid. In a phase diagram, pressure is plotted against temperature. The blue line is the boiling point. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. These diagrams indicate the physical states. Phase Diagram Labeled Boiling Point.
From www.chemistrylearner.com
Phase Diagram Definition, Explanation, and Diagram Phase Diagram Labeled Boiling Point The phase diagram below is for methane, ch 4. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. To be able to identify the triple point, the critical point, and four regions: The point where liquid become stable is called the triple point, where all three phases (solid, liquid. Phase Diagram Labeled Boiling Point.
From unistudium.unipg.it
Phase Diagrams Phase Diagram Labeled Boiling Point Notice that the boiling temperature changes a lot with a change in pressure. The phase diagram below is for methane, ch 4. Note, the axes are not drawn to. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. In a phase diagram, pressure is plotted against temperature. The solid. Phase Diagram Labeled Boiling Point.
From diagramtychow2z.z21.web.core.windows.net
Phase Diagram Normal Boiling Point Phase Diagram Labeled Boiling Point The solid green line shows the melting point of most liquids. Notice that the boiling temperature changes a lot with a change in pressure. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. Solid, liquid, gas, and a supercritical fluid. The blue line is the boiling point. The point. Phase Diagram Labeled Boiling Point.
From courses.lumenlearning.com
Phase Diagrams Chemistry Phase Diagram Labeled Boiling Point The phase diagram below is for methane, ch 4. The blue line is the boiling point. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The solid green line shows the melting point of most liquids. Notice that the boiling temperature changes a lot with a change in pressure.. Phase Diagram Labeled Boiling Point.
From www.chemistrylearner.com
Phase Diagram Definition, Explanation, and Diagram Phase Diagram Labeled Boiling Point In a phase diagram, pressure is plotted against temperature. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The phase diagram below is for methane, ch 4. Note, the. Phase Diagram Labeled Boiling Point.
From circuitwiringstroke123.z13.web.core.windows.net
Phase Diagram Labeled Boiling Point Phase Diagram Labeled Boiling Point The phase diagram below is for methane, ch 4. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. Note, the axes are not drawn to. The blue line is. Phase Diagram Labeled Boiling Point.
From civilengineeringcourses.com
Unary Phase Diagram Civil Engineering Courses Phase Diagram Labeled Boiling Point The blue line is the boiling point. Notice that the boiling temperature changes a lot with a change in pressure. Solid, liquid, gas, and a supercritical fluid. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. Note, the axes are not drawn to. In a phase diagram, pressure is. Phase Diagram Labeled Boiling Point.
From schematiclistangiomas.z14.web.core.windows.net
Phase Diagram Normal Boiling Point Phase Diagram Labeled Boiling Point The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Solid, liquid, gas, and a supercritical fluid. The phase diagram below is for methane, ch 4. Notice that the boiling temperature changes a lot with a change in pressure. These diagrams indicate the physical states that exist. Phase Diagram Labeled Boiling Point.
From mungfali.com
Phase Diagram Boiling Point Phase Diagram Labeled Boiling Point Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Notice that the boiling temperature changes a lot with a change in pressure. These diagrams indicate. Phase Diagram Labeled Boiling Point.
From guidemanualdragonized.z14.web.core.windows.net
Phase Diagram Labeled Boiling Point Phase Diagram Labeled Boiling Point The blue line is the boiling point. Notice that the boiling temperature changes a lot with a change in pressure. In a phase diagram, pressure is plotted against temperature. The solid green line shows the melting point of most liquids. Solid, liquid, gas, and a supercritical fluid. The point where liquid become stable is called the triple point, where all. Phase Diagram Labeled Boiling Point.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Phase Diagram Labeled Boiling Point The blue line is the boiling point. Note, the axes are not drawn to. To be able to identify the triple point, the critical point, and four regions: The solid green line shows the melting point of most liquids. Notice that the boiling temperature changes a lot with a change in pressure. These diagrams indicate the physical states that exist. Phase Diagram Labeled Boiling Point.
From galvinconanstuart.blogspot.com
Normal Boiling Point On Phase Diagram General Wiring Diagram Phase Diagram Labeled Boiling Point The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Solid, liquid, gas, and a supercritical fluid. Note, the axes are not drawn to. The blue line is the boiling point. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and. Phase Diagram Labeled Boiling Point.
From www.slideserve.com
PPT Freezing and Boiling Point Graph aka Phase Change Diagram or Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Solid, liquid, gas, and a supercritical fluid. The blue line is the boiling point. Note, the axes. Phase Diagram Labeled Boiling Point.
From wordpress.mrreid.org
temperature Phase Diagram Labeled Boiling Point To be able to identify the triple point, the critical point, and four regions: In a phase diagram, pressure is plotted against temperature. The blue line is the boiling point. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Notice that the boiling temperature changes a. Phase Diagram Labeled Boiling Point.
From jumpstarterdiscount.blogspot.com
Normal Boiling Point On Phase Diagram Wiring Diagram Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The phase diagram below is for methane, ch 4. Solid, liquid, gas, and a supercritical fluid. The blue line is the boiling point. Notice that the boiling temperature changes a lot with a change in pressure. The solid green line. Phase Diagram Labeled Boiling Point.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Phase Diagram Labeled Boiling Point Note, the axes are not drawn to. In a phase diagram, pressure is plotted against temperature. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. The phase diagram below is for methane, ch 4. These diagrams indicate the physical states that exist under specific conditions of. Phase Diagram Labeled Boiling Point.
From mavink.com
Phase Diagram Boiling Point Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. In a phase diagram, pressure is plotted against temperature. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Notice that the boiling temperature changes a lot. Phase Diagram Labeled Boiling Point.
From scienceisntscary.wordpress.com
Boiling point Ease Into Science Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The blue line is the boiling point. Notice that the boiling temperature changes a lot with a change in pressure.. Phase Diagram Labeled Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The phase diagram below is for methane, ch 4. The blue line is the boiling point. In a phase diagram, pressure is plotted against temperature. Note, the axes are not drawn to. Notice that the boiling temperature changes a lot. Phase Diagram Labeled Boiling Point.
From www.vrogue.co
Phase Diagram Normal Boiling Point vrogue.co Phase Diagram Labeled Boiling Point Notice that the boiling temperature changes a lot with a change in pressure. The solid green line shows the melting point of most liquids. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. These diagrams indicate the physical states that exist under specific conditions of pressure. Phase Diagram Labeled Boiling Point.
From partmcveighinjudicial.z21.web.core.windows.net
Normal Boiling Point On Phase Diagram Phase Diagram Labeled Boiling Point Solid, liquid, gas, and a supercritical fluid. The phase diagram below is for methane, ch 4. In a phase diagram, pressure is plotted against temperature. The solid green line shows the melting point of most liquids. The blue line is the boiling point. Notice that the boiling temperature changes a lot with a change in pressure. To be able to. Phase Diagram Labeled Boiling Point.
From unistudium.unipg.it
Phase Diagrams Phase Diagram Labeled Boiling Point These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. The blue line is the boiling point. The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. In a phase diagram, pressure is plotted against temperature. To. Phase Diagram Labeled Boiling Point.
From courses.lumenlearning.com
Phase Diagrams Chemistry Phase Diagram Labeled Boiling Point In a phase diagram, pressure is plotted against temperature. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure. Notice that the boiling temperature changes a lot with a change in pressure. The solid green line shows the melting point of most liquids. To be able to identify the triple. Phase Diagram Labeled Boiling Point.
From wirepartfrontwards.z14.web.core.windows.net
Phase Diagram Normal Boiling Point Phase Diagram Labeled Boiling Point The point where liquid become stable is called the triple point, where all three phases (solid, liquid and gas) are all in equilibrium. Note, the axes are not drawn to. The blue line is the boiling point. Solid, liquid, gas, and a supercritical fluid. In a phase diagram, pressure is plotted against temperature. These diagrams indicate the physical states that. Phase Diagram Labeled Boiling Point.