In A Titration Experiment Where Nh4Oh . This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Determine the reacting volumes of solutions of an acid and an alkali by titration. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Dilute with distilled water to about 100 ml. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. They then concentrate the solution and allow it to. Use the information to determine the concentration of the hydrochloric acid. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In each case, you start with 25 cm 3 of. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen.
from www.numerade.com
The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. Use the information to determine the concentration of the hydrochloric acid. Dilute with distilled water to about 100 ml. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. They then concentrate the solution and allow it to. This required practical involves using. Determine the reacting volumes of solutions of an acid and an alkali by titration. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. In each case, you start with 25 cm 3 of.
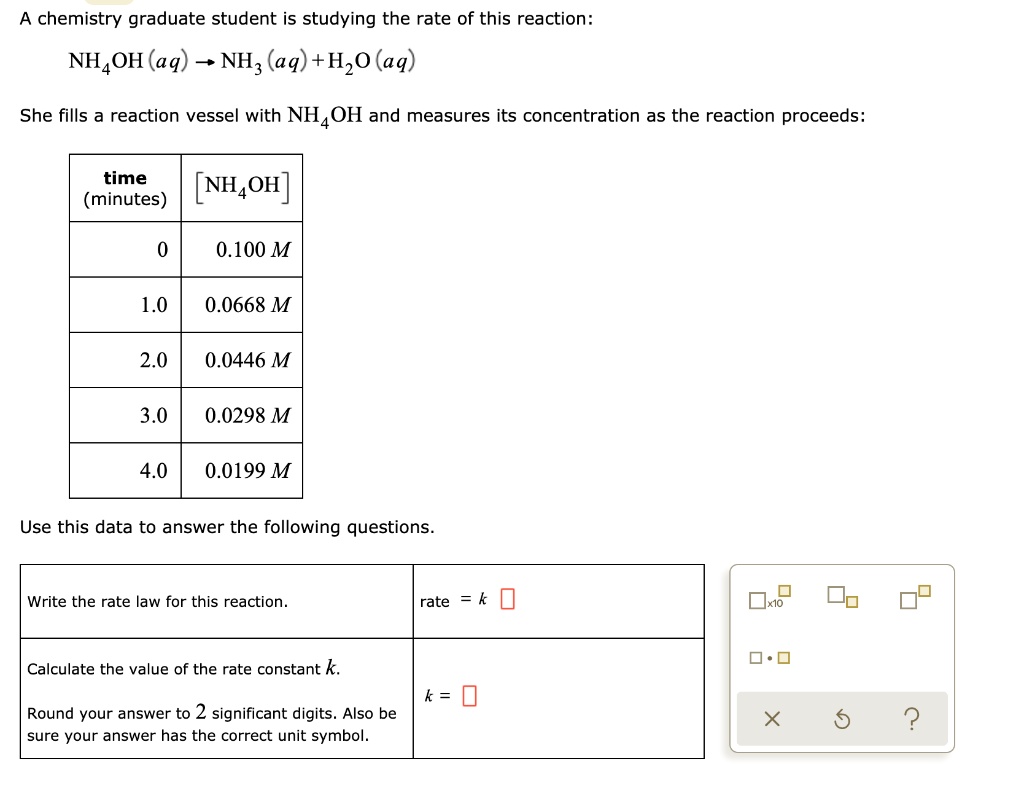
SOLVED A chemistry graduate student is studying the rate of this
In A Titration Experiment Where Nh4Oh A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. This required practical involves using. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. They then concentrate the solution and allow it to. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. In each case, you start with 25 cm 3 of. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Dilute with distilled water to about 100 ml. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Determine the reacting volumes of solutions of an acid and an alkali by titration. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Use the information to determine the concentration of the hydrochloric acid.
From www.shalom-education.com
Titrations Shalom Education In A Titration Experiment Where Nh4Oh A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Use the information to determine the concentration of the hydrochloric acid. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In each. In A Titration Experiment Where Nh4Oh.
From plot.ly
Weak Base (.10M NH4OH) and Strong Acid (.20M HCl) Titration scatter In A Titration Experiment Where Nh4Oh Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. Use the information to determine the concentration of the hydrochloric acid.. In A Titration Experiment Where Nh4Oh.
From www.sciencephoto.com
Titration experiment Stock Image C010/9622 Science Photo Library In A Titration Experiment Where Nh4Oh This required practical involves using. Dilute with distilled water to about 100 ml. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. In this experiment students neutralise sodium hydroxide. In A Titration Experiment Where Nh4Oh.
From chemistnotes.com
Conductometric titration, easy principle, curves, 3 advantages In A Titration Experiment Where Nh4Oh Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Use the information to determine the concentration of the hydrochloric acid. This required practical involves using. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. Determine the reacting volumes of solutions of an acid and an alkali by titration. A titration is. In A Titration Experiment Where Nh4Oh.
From www.meritnation.com
Concentration of NH4Cl and NH4OH in buffer solution is in the ratio 1 In A Titration Experiment Where Nh4Oh Dilute with distilled water to about 100 ml. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. They then concentrate the solution and allow it. In A Titration Experiment Where Nh4Oh.
From www.youtube.com
Conductometric Titrations NH4OH vs HCl YouTube In A Titration Experiment Where Nh4Oh The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Use the information to determine the concentration. In A Titration Experiment Where Nh4Oh.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube In A Titration Experiment Where Nh4Oh Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Determine the reacting volumes of solutions of an acid and an alkali by titration. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. They then concentrate the solution and allow it to. The amount of protein in a sample of cheese is determined by a kjeldahl. In A Titration Experiment Where Nh4Oh.
From theedge.com.hk
Chemistry How To Titration The Edge In A Titration Experiment Where Nh4Oh Use the information to determine the concentration of the hydrochloric acid. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. Determination of the reacting volumes of solutions of a. In A Titration Experiment Where Nh4Oh.
From www.numerade.com
SOLVED A chemistry graduate student is studying the rate of this In A Titration Experiment Where Nh4Oh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. Determine the reacting volumes of solutions of an acid and an alkali by titration. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. Use the information. In A Titration Experiment Where Nh4Oh.
From www.youtube.com
NH4OH+HCL 🧪Chemical experiment YouTube In A Titration Experiment Where Nh4Oh They then concentrate the solution and allow it to. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Use the information to determine the concentration of the hydrochloric acid. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. This required practical involves using. In each case, you start. In A Titration Experiment Where Nh4Oh.
From www.youtube.com
Titration (using phenolphthalein) YouTube In A Titration Experiment Where Nh4Oh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Use the information to determine the concentration of the hydrochloric acid. This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Pipette aliquot of hydrochloric acid solution into 250ml. In A Titration Experiment Where Nh4Oh.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry In A Titration Experiment Where Nh4Oh A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Dilute with distilled water to about 100 ml. Pipette aliquot of hydrochloric acid solution into 250ml. In A Titration Experiment Where Nh4Oh.
From acidsandbasesandmore.weebly.com
Titrations CHEMISTRY 101 In A Titration Experiment Where Nh4Oh A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The amount of. In A Titration Experiment Where Nh4Oh.
From www.chegg.com
Solved Titration experiments are done stepwise and slowly, In A Titration Experiment Where Nh4Oh The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. This required practical involves using. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Use the information to determine the concentration of the hydrochloric acid. A titration is an experiment where a volume of a solution of known concentration is added to. In A Titration Experiment Where Nh4Oh.
From www.alamy.com
Titration experiment, adding chemical of known concentration to test In A Titration Experiment Where Nh4Oh In each case, you start with 25 cm 3 of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. Dilute with distilled water. In A Titration Experiment Where Nh4Oh.
From www.embibe.com
The Plot of pHmetric titration of weak base NH4OH vs strong acid HCl In A Titration Experiment Where Nh4Oh Use the information to determine the concentration of the hydrochloric acid. Determine the reacting volumes of solutions of an acid and an alkali by titration. They then concentrate the solution and allow it to. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Pipette. In A Titration Experiment Where Nh4Oh.
From www.nagwa.com
Question Video Recalling the Correct Product Formed from the Reaction In A Titration Experiment Where Nh4Oh Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A 25 cm3 sample of hydrochloric acid is sucked. In A Titration Experiment Where Nh4Oh.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC In A Titration Experiment Where Nh4Oh A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the. In A Titration Experiment Where Nh4Oh.
From www.coursehero.com
[Solved] 1. Consider the titration experiment that yields the titration In A Titration Experiment Where Nh4Oh Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. Dilute with distilled water to about 100 ml. Determine the reacting volumes of solutions of an acid and an alkali by titration. A 25 cm3 sample of hydrochloric. In A Titration Experiment Where Nh4Oh.
From askfilo.com
In a titration experiment where NH4 OH is kept in the titration flask and.. In A Titration Experiment Where Nh4Oh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Use the information to determine the concentration of the hydrochloric acid. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Determine the reacting volumes of. In A Titration Experiment Where Nh4Oh.
From www.vecteezy.com
Acid base titration experiment and phases of color change during In A Titration Experiment Where Nh4Oh A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. Dilute. In A Titration Experiment Where Nh4Oh.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry In A Titration Experiment Where Nh4Oh They then concentrate the solution and allow it to. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. This required practical involves using. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. In this experiment students neutralise sodium hydroxide with. In A Titration Experiment Where Nh4Oh.
From www.slideserve.com
PPT Ammonia Titration Laboratory Activity The Problem PowerPoint In A Titration Experiment Where Nh4Oh The amount of protein in a sample of cheese is determined by a kjeldahl analysis for nitrogen. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. In A Titration Experiment Where Nh4Oh.
From mmerevise.co.uk
Titrations and Uncertainties MME In A Titration Experiment Where Nh4Oh Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In each case, you start with 25 cm 3 of. Determine the reacting volumes of solutions of an acid and an alkali by titration. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. This required practical involves using. They then concentrate the. In A Titration Experiment Where Nh4Oh.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS In A Titration Experiment Where Nh4Oh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Determine the reacting volumes of solutions of an acid and an alkali by titration. Dilute with distilled water to about 100 ml. A titration is an experiment where a volume of a solution of known concentration is added to a volume. In A Titration Experiment Where Nh4Oh.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC In A Titration Experiment Where Nh4Oh A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. Dilute with distilled water to about 100 ml. In each case, you start with 25 cm 3 of. This required practical involves using. In this experiment students neutralise sodium hydroxide with hydrochloric. In A Titration Experiment Where Nh4Oh.
From www.studocu.com
Double titration experiment Acidbase Titration using Method of In A Titration Experiment Where Nh4Oh A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. This required practical involves using. In each case, you start with 25 cm 3 of. Use the information to determine the concentration of the hydrochloric acid. They then concentrate the solution and allow it to. Dilute with distilled water to about 100 ml. Determination of the reacting. In A Titration Experiment Where Nh4Oh.
From studylib.net
Titration Lab In A Titration Experiment Where Nh4Oh A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. They then concentrate the solution and allow it to. Determine the reacting volumes of solutions of an acid and an alkali by titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. In A Titration Experiment Where Nh4Oh.
From letitsnowglobe.co.uk
Titration procedure pdf In A Titration Experiment Where Nh4Oh Use the information to determine the concentration of the hydrochloric acid. Determine the reacting volumes of solutions of an acid and an alkali by titration. In each case, you start with 25 cm 3 of. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. A titration is an experiment where a volume of a solution of known concentration is. In A Titration Experiment Where Nh4Oh.
From www.alamy.com
Adding NH4OH Ammonium Hydroxide to CuSO4 Copper II sulfate to Yield In A Titration Experiment Where Nh4Oh This required practical involves using. In each case, you start with 25 cm 3 of. Use the information to determine the concentration of the hydrochloric acid. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. Dilute with distilled water to about 100 ml. Determine the reacting volumes of solutions of an acid and an alkali by. In A Titration Experiment Where Nh4Oh.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog In A Titration Experiment Where Nh4Oh Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. They then concentrate the solution and. In A Titration Experiment Where Nh4Oh.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and In A Titration Experiment Where Nh4Oh They then concentrate the solution and allow it to. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. In each case, you start with 25 cm 3 of. Dilute with distilled water to about 100 ml. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A. In A Titration Experiment Where Nh4Oh.
From www.science-revision.co.uk
Titrations In A Titration Experiment Where Nh4Oh (higher tier only) determine the concentration of one of the solutions in mol/dm 3 and. A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. In each case, you start with 25 cm 3 of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Use the information. In A Titration Experiment Where Nh4Oh.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog In A Titration Experiment Where Nh4Oh A 25 cm3 sample of hydrochloric acid is sucked into a pipette and. They then concentrate the solution and allow it to. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to. In A Titration Experiment Where Nh4Oh.
From mmerevise.co.uk
Titrations and Uncertainties MME In A Titration Experiment Where Nh4Oh A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Determine the reacting volumes of solutions of an acid and an alkali by titration. Dilute with distilled water to about 100 ml. (higher tier only) determine the concentration of one of the solutions in mol/dm. In A Titration Experiment Where Nh4Oh.