What Size Container Do You Need To Hold 0 0459 Mol Of N2 . Pv = nrt p = 1 atm (stp) v = ? Created on may 16, 2022,. V = n x vm v = 0.0459 mol x 22.4. Your solution’s ready to go! Therefore, we can rearrange the formula to solve for v: The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. Calculate the volume of 0.0459 mol of n2 gas at stp. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? Our expert help has broken down your problem into an. What size container do you need to hold 0.0459 mol of n2 gas at stp? A molar quantity of a solid or liquid substance, will. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. What size container do you need to hold 0.0459 mol of n2 gas at stp?
from modugo.com
Our expert help has broken down your problem into an. What size container do you need to hold 0.0459 mol of n2 gas at stp? The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. V = n x vm v = 0.0459 mol x 22.4. Created on may 16, 2022,. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? Calculate the volume of 0.0459 mol of n2 gas at stp. Pv = nrt p = 1 atm (stp) v = ? What size container do you need to hold 0.0459 mol of n2 gas at stp? N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p.
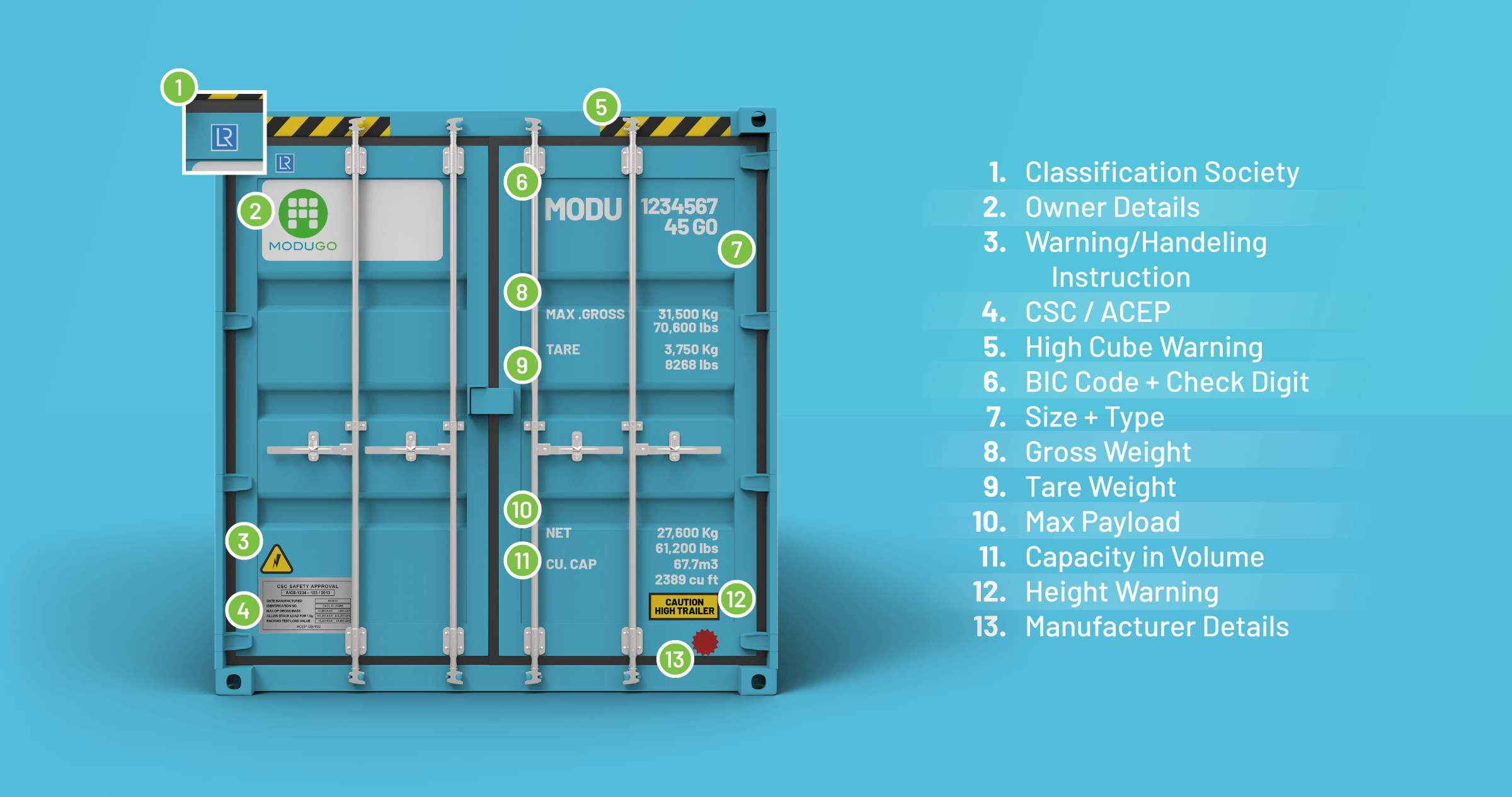
The Full Shipping Container Labels Guide ModuGo
What Size Container Do You Need To Hold 0 0459 Mol Of N2 Calculate the volume of 0.0459 mol of n2 gas at stp. Calculate the volume of 0.0459 mol of n2 gas at stp. What size container do you need to hold 0.0459 mol of n2 gas at stp? V = n x vm v = 0.0459 mol x 22.4. Created on may 16, 2022,. Our expert help has broken down your problem into an. Pv = nrt p = 1 atm (stp) v = ? Therefore, we can rearrange the formula to solve for v: Your solution’s ready to go! N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. A molar quantity of a solid or liquid substance, will. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. What size container do you need to hold 0.0459 mol of n2 gas at stp? What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp?
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 V = n x vm v = 0.0459 mol x 22.4. Our expert help has broken down your problem into an. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? The volume of the container needed to. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From dokumen.tips
(PPTX) 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 A molar quantity of a solid or liquid substance, will. Your solution’s ready to go! Our expert help has broken down your problem into an. Pv = nrt p = 1 atm (stp) v = ? What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? What size container do you need to hold. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.numerade.com
SOLVED 20. What size container do you need to hold 0.0459 Mol of N2 What Size Container Do You Need To Hold 0 0459 Mol Of N2 Pv = nrt p = 1 atm (stp) v = ? What size container do you need to hold 0.0459 mol of n2 gas at stp? Created on may 16, 2022,. V = n x vm v = 0.0459 mol x 22.4. Calculate the volume of 0.0459 mol of n2 gas at stp. Our expert help has broken down your. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 A molar quantity of a solid or liquid substance, will. Therefore, we can rearrange the formula to solve for v: Calculate the volume of 0.0459 mol of n2 gas at stp. What size container do you need to hold 0.0459 mol of n2 gas at stp? What size container do you need to hold 0.0459 mol of n2 gas at. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From oceanair.net
Size Matters How to Choose the Right Ocean Freight Container What Size Container Do You Need To Hold 0 0459 Mol Of N2 Calculate the volume of 0.0459 mol of n2 gas at stp. Therefore, we can rearrange the formula to solve for v: A molar quantity of a solid or liquid substance, will. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. Your solution’s ready to go! N = 0.0459 mol r = 0.0821 l·atm/mol·k t. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.container-xchange.com
Shipping container sizes [your 2022 guide to types & uses] What Size Container Do You Need To Hold 0 0459 Mol Of N2 The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. What size container do you need to hold 0.0459 mol of n2 gas at stp? What size container do you need to hold 0.0459 mol of n2 gas at stp? In this case, we know that n = 0.0459 mol and. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From mavink.com
Acetylene Cylinder Sizes Chart For Welding What Size Container Do You Need To Hold 0 0459 Mol Of N2 V = n x vm v = 0.0459 mol x 22.4. Therefore, we can rearrange the formula to solve for v: Your solution’s ready to go! A molar quantity of a solid or liquid substance, will. What size container do you need to hold 0.0459 mol of n2 gas at stp? What size container do you need to hold 0.0459. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.thefalconfreight.com
Reliable Freight Management Top Logistics Company What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. What size container do you need to hold 0.0459 mol of n2 gas at stp? Created on may 16, 2022,. V = n x vm v = 0.0459 mol x. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From slideplayer.com
Gases. ppt download What Size Container Do You Need To Hold 0 0459 Mol Of N2 Your solution’s ready to go! What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? A molar quantity of a solid or liquid substance, will. Calculate the volume of 0.0459 mol of n2 gas at stp. Created on may 16, 2022,. What size container do you need to hold 0.0459 mol of n2 gas. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.pinterest.com.au
CONTAINER SPECIFICATION CHARTS Shipping container sizes, Shipping What Size Container Do You Need To Hold 0 0459 Mol Of N2 Therefore, we can rearrange the formula to solve for v: V = n x vm v = 0.0459 mol x 22.4. Pv = nrt p = 1 atm (stp) v = ? The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. Calculate the volume of 0.0459 mol of n2 gas. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From williamwalsh.z13.web.core.windows.net
Plant Container Size Chart In Inches What Size Container Do You Need To Hold 0 0459 Mol Of N2 Therefore, we can rearrange the formula to solve for v: Created on may 16, 2022,. Our expert help has broken down your problem into an. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. A molar quantity of a solid or liquid substance, will. In this case, we know that n = 0.0459. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From cefcciem.blob.core.windows.net
Bin Vs Container at Antonio Walton blog What Size Container Do You Need To Hold 0 0459 Mol Of N2 Therefore, we can rearrange the formula to solve for v: In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. A molar quantity of a solid or liquid substance, will. Our expert help has broken down your problem into an. Calculate the volume of 0.0459 mol of n2 gas at stp. V = n x. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.picxsexy.com
A Guide To Shipping Container Sizes Big Box Container Porn Sex Picture What Size Container Do You Need To Hold 0 0459 Mol Of N2 Pv = nrt p = 1 atm (stp) v = ? Therefore, we can rearrange the formula to solve for v: What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? Our expert help has broken down your problem into an. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp). What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From vertainer.blogspot.com
Shipping container dimensions 10 foot HM What Size Container Do You Need To Hold 0 0459 Mol Of N2 In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. Calculate the volume of 0.0459 mol of n2 gas at stp. A molar quantity of a solid or liquid substance, will. Created on may 16, 2022,. Therefore, we can rearrange the formula to solve for v: Your solution’s ready to go! Our expert help has. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.pngkit.com
Download Shipping Container Dimensions Shipping Container Dimensions What Size Container Do You Need To Hold 0 0459 Mol Of N2 Created on may 16, 2022,. The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. Therefore, we can rearrange the formula to solve for v: What size container do you need to hold 0.0459 mol of n2 gas at stp? N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 Your solution’s ready to go! What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. A molar quantity of a solid or liquid substance, will. Therefore, we can rearrange the formula to solve for v: N = 0.0459 mol. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From slideplayer.com
QUESTION Consider the reaction N2 + 3 H2 2 NH3 Suppose mol of N2 is What Size Container Do You Need To Hold 0 0459 Mol Of N2 Calculate the volume of 0.0459 mol of n2 gas at stp. Pv = nrt p = 1 atm (stp) v = ? Our expert help has broken down your problem into an. Your solution’s ready to go! N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. What size container do you need to. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.securecontainer.ca
Shipping Container Dimensions & Sizes Secure Container What Size Container Do You Need To Hold 0 0459 Mol Of N2 Our expert help has broken down your problem into an. Pv = nrt p = 1 atm (stp) v = ? Created on may 16, 2022,. Therefore, we can rearrange the formula to solve for v: Calculate the volume of 0.0459 mol of n2 gas at stp. V = n x vm v = 0.0459 mol x 22.4. In this. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From fyokwbxmm.blob.core.windows.net
Mirage Container Measurements at June Goss blog What Size Container Do You Need To Hold 0 0459 Mol Of N2 Our expert help has broken down your problem into an. What size container do you need to hold 0.0459 mol of n2 gas at stp? The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. Pv = nrt p = 1 atm (stp) v = ? Therefore, we can rearrange the. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 Our expert help has broken down your problem into an. Pv = nrt p = 1 atm (stp) v = ? What size container do you need to hold 0.0459 mol of n2 gas at stp? V = n x vm v = 0.0459 mol x 22.4. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq}. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From pusan.skku.ac.kr
Want Standard Shipping Container Dimensions? [2023 Guide], 46 OFF What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? Calculate the volume of 0.0459 mol of n2 gas at stp. Pv = nrt p = 1 atm (stp) v = ? Our expert help has broken down your problem into an. What size container do you need to hold 0.0459 mol of n2 gas. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 Pv = nrt p = 1 atm (stp) v = ? Your solution’s ready to go! In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. N = 0.0459 mol r = 0.0821 l·atm/mol·k t =. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From exobdajhi.blob.core.windows.net
What Size Pot Do I Need For A Tree at Lourdes Moore blog What Size Container Do You Need To Hold 0 0459 Mol Of N2 The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. V = n x vm v = 0.0459 mol x 22.4. What size container do you need to hold 0.0459 mol of n2 gas at stp? Pv = nrt p = 1 atm (stp) v = ? What size container do. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.numerade.com
SOLVEDWhat size container do you need to hold 0.0459 mol of N2 gas at STP? What Size Container Do You Need To Hold 0 0459 Mol Of N2 Therefore, we can rearrange the formula to solve for v: Our expert help has broken down your problem into an. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. V = n x vm v = 0.0459 mol x 22.4. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.bigrentz.com
Storage Container Sizes Dimensions, Types and Cost BigRentz What Size Container Do You Need To Hold 0 0459 Mol Of N2 A molar quantity of a solid or liquid substance, will. Created on may 16, 2022,. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? Our expert help has broken down your problem into an. What size container. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT Entry Task April 13 th Friday PowerPoint Presentation, free What Size Container Do You Need To Hold 0 0459 Mol Of N2 Pv = nrt p = 1 atm (stp) v = ? Our expert help has broken down your problem into an. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. Calculate the volume of 0.0459 mol of n2. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.numerade.com
SOLVED What size container do you need to hold 0.0459 mol N2 gas at STP? What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? Calculate the volume of 0.0459 mol of n2 gas at stp. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. Our expert help has broken down your problem into an. V = n x vm v = 0.0459 mol. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.numerade.com
SOLVEDWhat size container do you need to hold 0.0459 mol of N2 gas at STP? What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? Therefore, we can rearrange the formula to solve for v: Pv = nrt p = 1 atm (stp) v = ? Our expert help has broken down your problem. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From cenxkqkd.blob.core.windows.net
Pot Sizes Chart at Pearl Crawford blog What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? A molar quantity of a solid or liquid substance, will. Our expert help has broken down your problem into an. In this case, we know that n = 0.0459. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. Created on may 16, 2022,. In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? A molar quantity of a solid or liquid. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From modugo.com
The Full Shipping Container Labels Guide ModuGo What Size Container Do You Need To Hold 0 0459 Mol Of N2 In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. N = 0.0459 mol r = 0.0821 l·atm/mol·k t = 273 k (stp) v = nrt/p. Your solution’s ready to go! What size container do you. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From exyagadjk.blob.core.windows.net
Cargo Container Sizes at Robert Dawson blog What Size Container Do You Need To Hold 0 0459 Mol Of N2 In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. Therefore, we can rearrange the formula to solve for v: Created on may 16, 2022,. A molar quantity of a solid or liquid substance, will. The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. What. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.chegg.com
Solved A mixture of 0.50 mol H2(g) and 0.7 mol N2(g) is What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. A molar quantity of a solid or liquid substance, will. Our expert help has broken down your problem into an. What size container do you need. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.slideserve.com
PPT 20. What size container do you need to hole 0.0459 mol of N2 gas What Size Container Do You Need To Hold 0 0459 Mol Of N2 What size container do you need to hold 0.0459 mol of n2 gas at stp? Created on may 16, 2022,. What size container do you need to hold 0.0459 mol of n2 gas at stp? In this case, we know that n = 0.0459 mol and vm = 22.4 l/mol. N = 0.0459 mol r = 0.0821 l·atm/mol·k t =. What Size Container Do You Need To Hold 0 0459 Mol Of N2.
From www.discovercontainers.com
Shipping Container Dimensions and Sizes Discover Containers What Size Container Do You Need To Hold 0 0459 Mol Of N2 The volume of the container needed to hold 0.0459 mol of n₂ gas at stp is approximately 1.029 l. Created on may 16, 2022,. V = n x vm v = 0.0459 mol x 22.4. What size container do you need to hold 0.0459 mole of {eq}n_2 {/eq} gas at stp? What size container do you need to hold 0.0459. What Size Container Do You Need To Hold 0 0459 Mol Of N2.