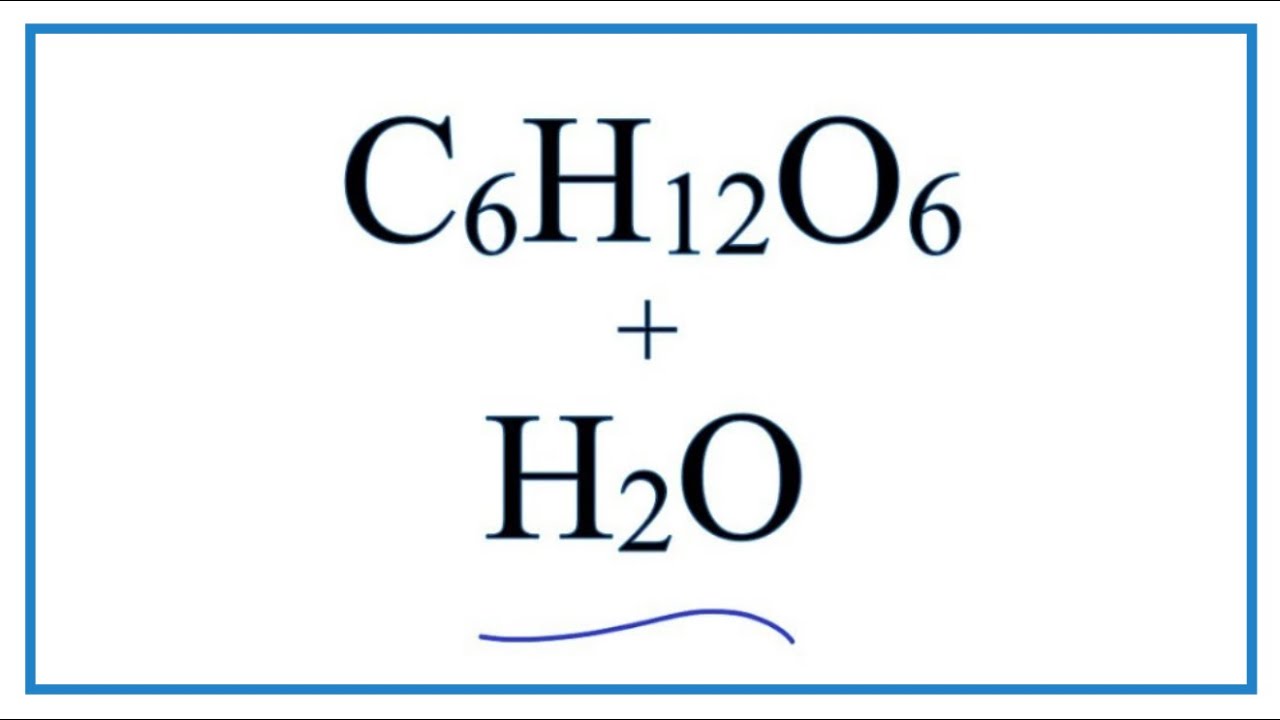
Sugar Chemical Water . Is dissolving sugar in water an example of a chemical or physical change? When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Then gently swirl the liquid and m&m in each cup for about 30 seconds. Here are the answer and an explanation of the process. At the same time, add 1 m&m to each liquid. The solubility of sugar in vinegar is negligible; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. It takes energy to break the bonds. But sugar does dissolve in water, and this. We have presented a microscopic view of the chemical reaction between oxygen and hydrogen.
from dxobuticl.blob.core.windows.net
At the same time, add 1 m&m to each liquid. But sugar does dissolve in water, and this. We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. The solubility of sugar in vinegar is negligible; Here are the answer and an explanation of the process. It takes energy to break the bonds. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Is dissolving sugar in water an example of a chemical or physical change? Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent.
Sugar Water Chemical Formula at Savannah Osgood blog
Sugar Chemical Water Then gently swirl the liquid and m&m in each cup for about 30 seconds. At the same time, add 1 m&m to each liquid. We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Here are the answer and an explanation of the process. But sugar does dissolve in water, and this. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. The solubility of sugar in vinegar is negligible; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Then gently swirl the liquid and m&m in each cup for about 30 seconds. It takes energy to break the bonds. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Is dissolving sugar in water an example of a chemical or physical change?
From www.thoughtco.com
What Is the Chemical Formula of Sugar? Sugar Chemical Water Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Here are the answer and an explanation of the process. But sugar does dissolve in water, and this. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: When sugar dissolves in water, the weak bonds between the individual sucrose. Sugar Chemical Water.
From www.slideserve.com
PPT BODY OF KNOWLEDGE PowerPoint Presentation ID2125761 Sugar Chemical Water But sugar does dissolve in water, and this. Then gently swirl the liquid and m&m in each cup for about 30 seconds. We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. It takes energy to break the bonds. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these. Sugar Chemical Water.
From techiescientist.com
Is Sugar Water Homogeneous or Heterogeneous? Techiescientist Sugar Chemical Water Then gently swirl the liquid and m&m in each cup for about 30 seconds. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Is dissolving sugar in water an example of a chemical or physical change? We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. It takes energy. Sugar Chemical Water.
From www.youtube.com
Dissolving Sugar in Water YouTube Sugar Chemical Water But sugar does dissolve in water, and this. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Then gently swirl the liquid and m&m in each cup for about 30 seconds. It takes energy to break the bonds. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken,. Sugar Chemical Water.
From brainly.com
The diagram below represents the creation of a sugar solution by adding Sugar Chemical Water It takes energy to break the bonds. The solubility of sugar in vinegar is negligible; Here are the answer and an explanation of the process. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Is dissolving sugar in water an example. Sugar Chemical Water.
From www.reagent.co.uk
The Chemistry Behind Sugar ReAgent Chemical Services Sugar Chemical Water We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. The solubility of sugar in vinegar is negligible; It takes energy to break the bonds. Then gently swirl the liquid and m&m in each cup for about 30 seconds. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Is. Sugar Chemical Water.
From chemistryiitjamnetset.blogspot.com
IIT JAM UGC CSIR NET GATE CHEMISTRY Reaction of sugar with sulfuric acid Sugar Chemical Water Then gently swirl the liquid and m&m in each cup for about 30 seconds. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Is dissolving sugar in water an example of a chemical or physical change? We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Acetic acid in solution and. Sugar Chemical Water.
From www.biologyonline.com
Reducing sugar Definition and Examples Biology Online Dictionary Sugar Chemical Water But sugar does dissolve in water, and this. It takes energy to break the bonds. The solubility of sugar in vinegar is negligible; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Here are the answer and an explanation of the process. Is dissolving sugar in water an example of a chemical or physical change?. Sugar Chemical Water.
From www.youtube.com
Sugar In Water YouTube Sugar Chemical Water Here are the answer and an explanation of the process. But sugar does dissolve in water, and this. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Then gently swirl the liquid and m&m in each cup for about 30 seconds.. Sugar Chemical Water.
From www.scienceabc.com
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC Sugar Chemical Water But sugar does dissolve in water, and this. Here are the answer and an explanation of the process. Then gently swirl the liquid and m&m in each cup for about 30 seconds. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Is dissolving sugar in water an example of a chemical or physical change? The. Sugar Chemical Water.
From www.cookist.com
Everything You Need To Know About Sugar Water — Are They Really Better Sugar Chemical Water When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Here are the answer and an explanation of the process. It takes energy to break the bonds. The solubility of sugar in vinegar is negligible; Is dissolving sugar in water an example. Sugar Chemical Water.
From bettertogether.org
Is Salt Dissolving In Water A Chemical Change Better Together Sugar Chemical Water It takes energy to break the bonds. Here are the answer and an explanation of the process. The solubility of sugar in vinegar is negligible; When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Then gently swirl the liquid and m&m. Sugar Chemical Water.
From sciencing.com
How to Separate a Mixture of Sugar & Water Sciencing Sugar Chemical Water Is dissolving sugar in water an example of a chemical or physical change? Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. But sugar does dissolve in water, and this. Then gently swirl the liquid and m&m in each cup for about 30 seconds. When sugar dissolves in water, the weak bonds between. Sugar Chemical Water.
From www.sugar.org
Sugar is an Environmentally Friendly Household Product The Sugar Sugar Chemical Water Then gently swirl the liquid and m&m in each cup for about 30 seconds. But sugar does dissolve in water, and this. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. We have presented a microscopic view of the chemical reaction. Sugar Chemical Water.
From www.nutriinspector.com
Is Sugar Dissolving in Water a Chemical Change? Sugar Chemical Water Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: But sugar does dissolve in water, and this. We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Then gently swirl the liquid and m&m in. Sugar Chemical Water.
From stock.adobe.com
Chemical illustration. The solubility of sugar and water. Sugar is the Sugar Chemical Water Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Then gently swirl the liquid and m&m in each cup for about 30 seconds. Here are the answer and an explanation of the process. The solubility of sugar in vinegar. Sugar Chemical Water.
From dxobuticl.blob.core.windows.net
Sugar Water Chemical Formula at Savannah Osgood blog Sugar Chemical Water Is dissolving sugar in water an example of a chemical or physical change? Then gently swirl the liquid and m&m in each cup for about 30 seconds. The solubility of sugar in vinegar is negligible; When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are. Sugar Chemical Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Sugar Chemical Water When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. We have. Sugar Chemical Water.
From chemistry-europe.onlinelibrary.wiley.com
Regulating Antifreeze Activity through Water Latent Functions of the Sugar Chemical Water We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. The solubility of sugar in vinegar is negligible; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from. Sugar Chemical Water.
From www.redbubble.com
"Sugar Chemical Structure of Glucose" by theelements Redbubble Sugar Chemical Water The solubility of sugar in vinegar is negligible; When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: But sugar does dissolve in water, and this. We have. Sugar Chemical Water.
From www.slideserve.com
PPT Biochemistry of Cells PowerPoint Presentation, free download ID Sugar Chemical Water When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Is dissolving sugar in water an example of a chemical or physical change? We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Sugar has the chemical formulate. Sugar Chemical Water.
From www.thoughtco.com
What Is the Chemical Formula of Sugar? Sugar Chemical Water Here are the answer and an explanation of the process. Then gently swirl the liquid and m&m in each cup for about 30 seconds. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. It takes energy to break the bonds. Is. Sugar Chemical Water.
From www.dreamstime.com
Glucose. Chemical Structural Formula and Model of Molecule. C6H12O6 Sugar Chemical Water It takes energy to break the bonds. But sugar does dissolve in water, and this. At the same time, add 1 m&m to each liquid. Here are the answer and an explanation of the process. The solubility of sugar in vinegar is negligible; Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Sugar. Sugar Chemical Water.
From study.com
Sugar Molecule Structure & Formula Video & Lesson Transcript Sugar Chemical Water When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. It takes energy to break the bonds. Here are the answer and an explanation of the. Sugar Chemical Water.
From www.nutriinspector.com
Is Sugar Dissolving in Water a Chemical Change? Sugar Chemical Water At the same time, add 1 m&m to each liquid. The solubility of sugar in vinegar is negligible; Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Is dissolving sugar in water an example of a chemical or physical change? Here are the answer and an explanation of the process. When sugar dissolves. Sugar Chemical Water.
From chemistrynerds963.blogspot.com
SUGAR MOLECULES/CHEMICAL FORMULA Sugar Chemical Water We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. Is dissolving sugar in water an example of a chemical or physical change? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: But sugar does. Sugar Chemical Water.
From www.pro-factory-plus.eu
Molecular Formula Of Sugar Water ProFactoryPlus Perspective Sugar Chemical Water The solubility of sugar in vinegar is negligible; We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. Here are the answer and an explanation of the process. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: But sugar does dissolve in water, and this. At the same time, add 1. Sugar Chemical Water.
From www.thoughtco.com
Dissolving Sugar in Water Chemical or Physical Change? Sugar Chemical Water Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. The solubility of sugar in vinegar is negligible; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o. Sugar Chemical Water.
From www.sugar.org
What is Sugar? What is Sucrose? The Sugar Association Sugar Chemical Water Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. At the same time, add 1 m&m to each liquid. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. But sugar does dissolve in water,. Sugar Chemical Water.
From www.thoughtco.com
Dissolving Sugar in Water Chemical or Physical Change? Sugar Chemical Water Is dissolving sugar in water an example of a chemical or physical change? At the same time, add 1 m&m to each liquid. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. But sugar does dissolve in water, and this. Then. Sugar Chemical Water.
From www.alamy.com
Undissolved sugar hires stock photography and images Alamy Sugar Chemical Water We have presented a microscopic view of the chemical reaction between oxygen and hydrogen. The solubility of sugar in vinegar is negligible; When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Is dissolving sugar in water an example of a chemical. Sugar Chemical Water.
From www.tessshebaylo.com
Balanced Equation Of Sucrose And Sulfuric Acid Tessshebaylo Sugar Chemical Water Then gently swirl the liquid and m&m in each cup for about 30 seconds. Is dissolving sugar in water an example of a chemical or physical change? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: It takes energy to break the bonds. But sugar does dissolve in water, and this. Here are the answer. Sugar Chemical Water.
From www.slideserve.com
PPT Natural Approach to Chemistry Chapter 9 Water & Solutions Sugar Chemical Water When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. At the same time, add 1 m&m to each liquid. But sugar does dissolve in water,. Sugar Chemical Water.
From fphoto.photoshelter.com
science chemistry mixture solution Fundamental Photographs The Art Sugar Chemical Water The solubility of sugar in vinegar is negligible; Is dissolving sugar in water an example of a chemical or physical change? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: But sugar does dissolve in water, and this. Acetic acid in solution and sucrose do not undergo a chemical reaction to any appreciable extent. At. Sugar Chemical Water.
From worldwaterforum7.org
Is Sugar Dissolving in Water a Chemical or a Physical Change? WWF7 Sugar Chemical Water Is dissolving sugar in water an example of a chemical or physical change? When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these c 12 h 22 o 11 molecules are released into solution. Then gently swirl the liquid and m&m in each cup for about 30 seconds. Here are the answer and. Sugar Chemical Water.