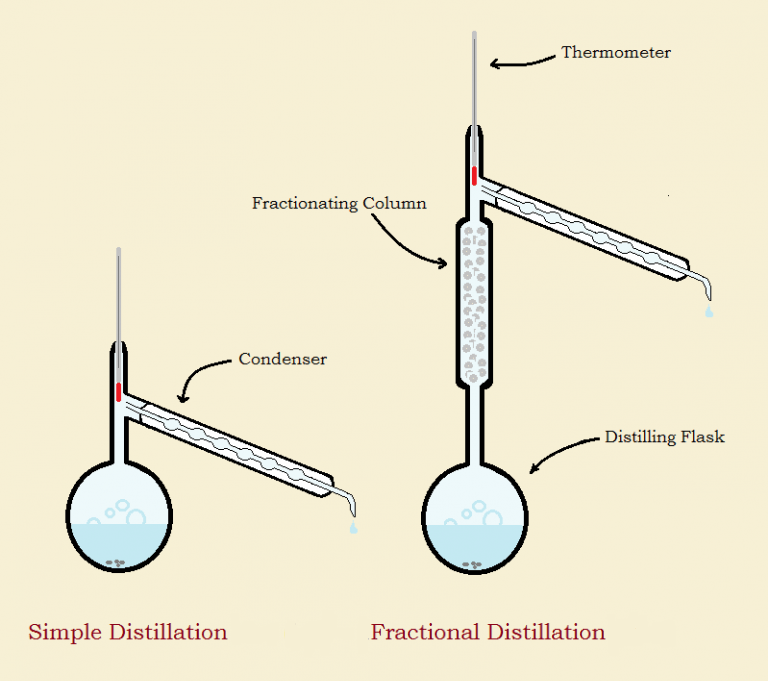
Distillation Example Chemistry . In this lab tutorial, we discuss simple distillation, its. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. under simple distillation, this means that the lower boiling point component has already finished distilling.
from www.quirkyscience.com
describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. under simple distillation, this means that the lower boiling point component has already finished distilling. In this lab tutorial, we discuss simple distillation, its. Learn more in this ks3 chemistry guide from bitesize. distillation is a separation technique used to remove a solvent from a mixture and keep it. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation refers to the selective boiling and subsequent condensation of a component in a liquid.
Chemical Separation by Fractional Distillation and Crystallization
Distillation Example Chemistry distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. under simple distillation, this means that the lower boiling point component has already finished distilling. In this lab tutorial, we discuss simple distillation, its.
From www.youtube.com
What is Simple Distillation Separation Methods Chemistry Basics YouTube Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a separation technique used to remove a solvent from a mixture and keep it. under simple distillation, this means that the lower boiling. Distillation Example Chemistry.
From www.dreamstime.com
Distillation Vector Illustration. Liquid Substance Separation Explanation. Stock Vector Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation is a separation technique used to remove a solvent from a mixture and keep it. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. under simple distillation, this means that the lower boiling. Distillation Example Chemistry.
From www.shutterstock.com
diagramme de distillation simple en chimie image vectorielle de stock (libre de droits Distillation Example Chemistry distillation is a separation technique used to remove a solvent from a mixture and keep it. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. Learn more in this ks3 chemistry guide from bitesize. In this lab tutorial, we discuss simple. Distillation Example Chemistry.
From www.thoughtco.com
What Is Distillation? Principles and Uses Distillation Example Chemistry distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. In this lab tutorial, we discuss simple distillation, its.. Distillation Example Chemistry.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Distillation Example Chemistry To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. Learn more in this ks3 chemistry guide from bitesize. distillation is a separation technique used to remove a solvent from a mixture and keep it. under simple distillation, this means that the lower boiling point component has already finished distilling. In. Distillation Example Chemistry.
From www.shalom-education.com
Simple and Fractional Distillation KS3 Chemistry Revision Distillation Example Chemistry under simple distillation, this means that the lower boiling point component has already finished distilling. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. To separate the various hydrocarbons and. Distillation Example Chemistry.
From www.vecteezy.com
Simple distillation model in chemistry laboratory 2399309 Vector Art at Vecteezy Distillation Example Chemistry distillation is a separation technique used to remove a solvent from a mixture and keep it. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. In this lab tutorial, we discuss simple distillation, its. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried. Distillation Example Chemistry.
From www.youtube.com
Steam Distillation Technique Basic Principles and Techniques in Organic Chemistry YouTube Distillation Example Chemistry distillation is a separation technique used to remove a solvent from a mixture and keep it. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to. Distillation Example Chemistry.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier Distillation Example Chemistry under simple distillation, this means that the lower boiling point component has already finished distilling. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. distillation is a separation technique used to remove a solvent from a mixture and keep it. In this lab tutorial, we discuss simple distillation, its.. Distillation Example Chemistry.
From www.youtube.com
Animated Water Distillation process diagram in PowerPoint / Chemistry Classes / Free PPT YouTube Distillation Example Chemistry In this lab tutorial, we discuss simple distillation, its. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. describe the role of distillation in crude. Distillation Example Chemistry.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. In this lab tutorial, we discuss simple distillation, its. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows.. Distillation Example Chemistry.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. under simple distillation, this means that the lower boiling point component has already finished distilling. Learn. Distillation Example Chemistry.
From mavink.com
Labelled Diagram Of Distillation Distillation Example Chemistry under simple distillation, this means that the lower boiling point component has already finished distilling. In this lab tutorial, we discuss simple distillation, its. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. describe the role of distillation in crude oil refining, and explain, in a very general way, how. Distillation Example Chemistry.
From issr.edu.kh
Distillation Distillation Example Chemistry describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. Learn more in this ks3 chemistry guide from bitesize. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. To separate the various hydrocarbons and petroleum derivatives,. Distillation Example Chemistry.
From in.pinterest.com
Distillation Process Science HowThingsWork Gif Distillation process, Distillation, Process Distillation Example Chemistry To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. under simple distillation, this means that the lower boiling point component has already finished distilling.. Distillation Example Chemistry.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous (Tanpa Royalti) 1950051133 Distillation Example Chemistry distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. In this lab tutorial, we discuss simple distillation, its. Learn more in this ks3 chemistry guide from bitesize. distillation, process involving the. Distillation Example Chemistry.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Distillation Example Chemistry To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. In this lab tutorial, we discuss simple distillation, its. distillation refers to the selective boiling and subsequent condensation of a component in. Distillation Example Chemistry.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Distillation Example Chemistry describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows.. Distillation Example Chemistry.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Distillation Example Chemistry Learn more in this ks3 chemistry guide from bitesize. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. distillation is a purification method for liquids, and can separate components of a mixture if. Distillation Example Chemistry.
From chem.libretexts.org
5.3D StepbyStep Procedures for Fractional Distillation Chemistry LibreTexts Distillation Example Chemistry In this lab tutorial, we discuss simple distillation, its. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. Learn more in this ks3 chemistry guide from bitesize. under simple distillation, this means that the lower boiling point component has already finished distilling. distillation is a separation technique used to. Distillation Example Chemistry.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Example Chemistry To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to. Distillation Example Chemistry.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Distillation Example Chemistry under simple distillation, this means that the lower boiling point component has already finished distilling. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. In this lab tutorial, we discuss simple distillation, its. distillation is a purification method for liquids, and can separate components of a mixture if they have. Distillation Example Chemistry.
From www.slideserve.com
PPT Separation Techniques Grade 10 Chemistry PowerPoint Presentation ID270438 Distillation Example Chemistry In this lab tutorial, we discuss simple distillation, its. Learn more in this ks3 chemistry guide from bitesize. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. distillation refers to. Distillation Example Chemistry.
From de.wikipedia.org
Destillation Wikipedia Distillation Example Chemistry distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. distillation, process involving the conversion of a liquid into vapour that is subsequently. Distillation Example Chemistry.
From www.youtube.com
Distillation Definition and Technique Basic Principles and Techniques in Organic Chemistry Distillation Example Chemistry Learn more in this ks3 chemistry guide from bitesize. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation is a separation technique used to remove a solvent from a mixture and. Distillation Example Chemistry.
From www.geeksforgeeks.org
Distillation Definition, Meaning, Principle, Types & Uses Distillation Example Chemistry distillation is a separation technique used to remove a solvent from a mixture and keep it. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation refers to the selective boiling and subsequent condensation of a component in a liquid.. Distillation Example Chemistry.
From www.pinterest.com
Figure 1 Fractional distillation apparatus using a Liebig condenser. Fractional distillation Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. . Distillation Example Chemistry.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Distillation Example Chemistry Learn more in this ks3 chemistry guide from bitesize. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. In this lab tutorial, we discuss. Distillation Example Chemistry.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Distillation Example Chemistry describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. In this lab tutorial, we discuss simple distillation, its. distillation refers to the selective. Distillation Example Chemistry.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. under simple distillation, this means that the lower boiling point component has already finished distilling. distillation is a separation technique used to remove a solvent from a mixture and keep it. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation. Distillation Example Chemistry.
From chemistnotes.com
Distillation Definition and Types of Distillation Chemistry Notes Distillation Example Chemistry distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to. Distillation Example Chemistry.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Distillation Example Chemistry describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. Learn more in this ks3 chemistry guide from bitesize. under simple distillation, this means. Distillation Example Chemistry.
From www.dreamstime.com
Simple Distillation Apparatus Diagram with Full Process Vector Illustration Stock Vector Distillation Example Chemistry distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. describe the role of distillation. Distillation Example Chemistry.
From www.quirkyscience.com
Chemical Separation by Fractional Distillation and Crystallization Distillation Example Chemistry Learn more in this ks3 chemistry guide from bitesize. distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid. describe the role of distillation in crude oil refining, and explain, in a very general way, how further processing is used to increase the yield of. In this lab tutorial, we discuss. Distillation Example Chemistry.
From www.scienceabc.com
Denatured Alcohol Definition, Properties, Examples And Uses Distillation Example Chemistry To separate the various hydrocarbons and petroleum derivatives, a fractional distillation method is carried out that allows. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. In this lab tutorial, we discuss simple distillation, its. under simple distillation, this means that the lower boiling point component has already. Distillation Example Chemistry.