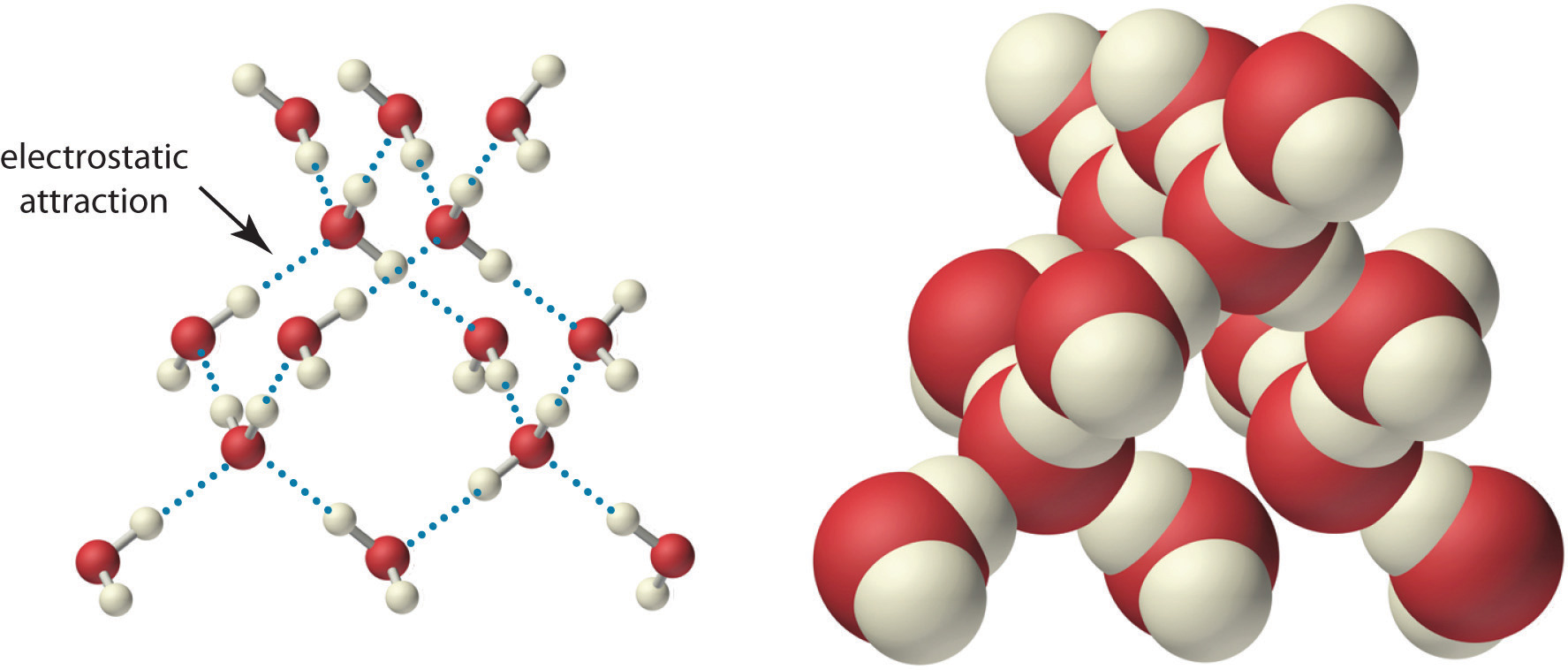
What Is Liquid Water Structure . Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. The values of water's heat of fusion (5.98.
from saylordotorg.github.io
The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. The values of water's heat of fusion (5.98. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor.
Aqueous Solutions
What Is Liquid Water Structure Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Because of the higher electronegativity of the oxygen atom, the bonds are polar. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The values of water's heat of fusion (5.98. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds.
From www.universallifetools.com
Liquid Crystalline Body Structured Water What Is Liquid Water Structure In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The values of water's heat of fusion (5.98. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers. What Is Liquid Water Structure.
From www.sciencephoto.com
Molecular structure of liquid water Stock Image A504/0088 Science What Is Liquid Water Structure Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The values of water's heat of fusion (5.98. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and.. What Is Liquid Water Structure.
From www.universallifetools.com
Liquid Crystalline Body Structured Water What Is Liquid Water Structure Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. The values of water's heat of fusion (5.98. The structure of liquid water is very similar, but in the. What Is Liquid Water Structure.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Water and pH What Is Liquid Water Structure In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. The structure. What Is Liquid Water Structure.
From mavink.com
The Structure Of Water What Is Liquid Water Structure The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. The. What Is Liquid Water Structure.
From ar.inspiredpencil.com
Liquid Water Molecular Structure What Is Liquid Water Structure Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Water is a simple molecule consisting. What Is Liquid Water Structure.
From webmis.highland.cc.il.us
Aqueous Solutions What Is Liquid Water Structure Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Because of the higher electronegativity of the oxygen atom, the bonds are polar. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Liquid water has. What Is Liquid Water Structure.
From www.thoughtco.com
Liquid Definition and Examples (Chemistry) What Is Liquid Water Structure Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. The values of water's heat of fusion (5.98. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as. What Is Liquid Water Structure.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, What Is Liquid Water Structure Because of the higher electronegativity of the oxygen atom, the bonds are polar. The values of water's heat of fusion (5.98. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are. What Is Liquid Water Structure.
From saylordotorg.github.io
Aqueous Solutions What Is Liquid Water Structure Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form. What Is Liquid Water Structure.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Is Liquid Water Structure In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Water is what wets windows when it rains, what we drink when we feel thirsty,. What Is Liquid Water Structure.
From www.sciencephoto.com
Water and ice molecular structure, illustration Stock Image C035 What Is Liquid Water Structure In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. The structure. What Is Liquid Water Structure.
From www.alamy.com
Chemical structure of a water molecule, H2O Stock Photo Alamy What Is Liquid Water Structure In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Water is liquid at room temperature. What Is Liquid Water Structure.
From www.learnz.org.nz
The Water Cycle LEARNZ What Is Liquid Water Structure Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. In reality, the fact that liquid water. What Is Liquid Water Structure.
From www.scienceabc.com
Why Does Water Expand When It Freezes? » ScienceABC What Is Liquid Water Structure Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. The structure of liquid water is very similar, but in the. What Is Liquid Water Structure.
From alevelbiology.co.uk
Dipoles Of Water Molecules ALevel Biology Revision Notes What Is Liquid Water Structure Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid,. What Is Liquid Water Structure.
From drmchemistrytutor.com
Hydrogen Bonding in water Dr. M. Chemistry Tutor What Is Liquid Water Structure Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Liquid water has importance as a solvent,. What Is Liquid Water Structure.
From www.snexplores.org
Explainer What are the different states of matter? What Is Liquid Water Structure Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Liquid water has importance as a solvent, a solute, a. What Is Liquid Water Structure.
From www.slideserve.com
PPT Water and Its Properties PowerPoint Presentation, free download What Is Liquid Water Structure Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. The values of water's heat of fusion (5.98. Water is a simple. What Is Liquid Water Structure.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure What Is Liquid Water Structure The values of water's heat of fusion (5.98. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Water is what wets. What Is Liquid Water Structure.
From saylordotorg.github.io
Liquids What Is Liquid Water Structure Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Hydrogen bond formation requires both a hydrogen. What Is Liquid Water Structure.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning What Is Liquid Water Structure Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. The values of water's heat of fusion (5.98. Liquid water has. What Is Liquid Water Structure.
From alevelbiology.co.uk
Water Structure And Properties Molecule & Physical Properties A Level What Is Liquid Water Structure Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Liquid water is unique for its high specific heat capacity, surface tension, and ability. What Is Liquid Water Structure.
From www.dreamstime.com
Water States of Matter Phase. Change of State for Water Diagram Stock What Is Liquid Water Structure Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of earth's surface. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor.. What Is Liquid Water Structure.
From www.researchgate.net
(A) Schematic representation of the liquid water structure and the What Is Liquid Water Structure Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules. What Is Liquid Water Structure.
From www.slideserve.com
PPT Water and Its Properties PowerPoint Presentation, free download What Is Liquid Water Structure Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. The values of water's heat of fusion (5.98. Liquid water has importance as a solvent, a solute,. What Is Liquid Water Structure.
From ar.inspiredpencil.com
Liquid Water Structure What Is Liquid Water Structure In reality, the fact that liquid water is denser than ice is responsible for the floating of ice. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Hydrogen bond formation. What Is Liquid Water Structure.
From worldoceanreview.com
Water a unique molecule « World Ocean Review What Is Liquid Water Structure Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of. What Is Liquid Water Structure.
From www.universallifetools.com
Liquid Crystalline Body Structured Water What Is Liquid Water Structure The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The values of water's heat of fusion (5.98. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor.. What Is Liquid Water Structure.
From www.i-sis.org.uk
What is Liquid Water? What Is Liquid Water Structure Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Water is what wets windows when it. What Is Liquid Water Structure.
From nicholasbsullivan.com
Images and Figures for Oceanography What Is Liquid Water Structure Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid,. What Is Liquid Water Structure.
From www.visionlearning.com
Water Chemistry Visionlearning What Is Liquid Water Structure Because of the higher electronegativity of the oxygen atom, the bonds are polar. Hydrogen bond formation requires both a hydrogen bond donor and a hydrogen bond acceptor. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Water is what wets windows when it rains, what we drink when we feel thirsty, and what. What Is Liquid Water Structure.
From ar.inspiredpencil.com
Liquid Water Molecular Structure What Is Liquid Water Structure Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is what wets windows when it rains, what we drink when we feel thirsty, and what covers about 70% of. What Is Liquid Water Structure.
From childhealthpolicy.vumc.org
🌷 7 properties of water. 9 Properties of Water along with Examples What Is Liquid Water Structure Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the. Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. In reality, the fact that liquid water is denser than. What Is Liquid Water Structure.
From ecampusontario.pressbooks.pub
13.5 Water A Special Liquid Enhanced Introductory College Chemistry What Is Liquid Water Structure Liquid water has importance as a solvent, a solute, a reactant, a catalyst, and a biomolecule, structuring proteins, nucleic acid, and cells and. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer. What Is Liquid Water Structure.