Copper Chloride Zinc Equation . However, in the second reaction, the zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. the equation for the reaction is. write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. 2 cucl2 → 2 cucl + cl2. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. this is a redox reaction in which the oxidation states of the metals clearly change.
from www.chegg.com
zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. 2 cucl2 → 2 cucl + cl2. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. this is a redox reaction in which the oxidation states of the metals clearly change. in the first reaction, the copper ion is able to oxidize the zinc metal. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. However, in the second reaction, the zinc.
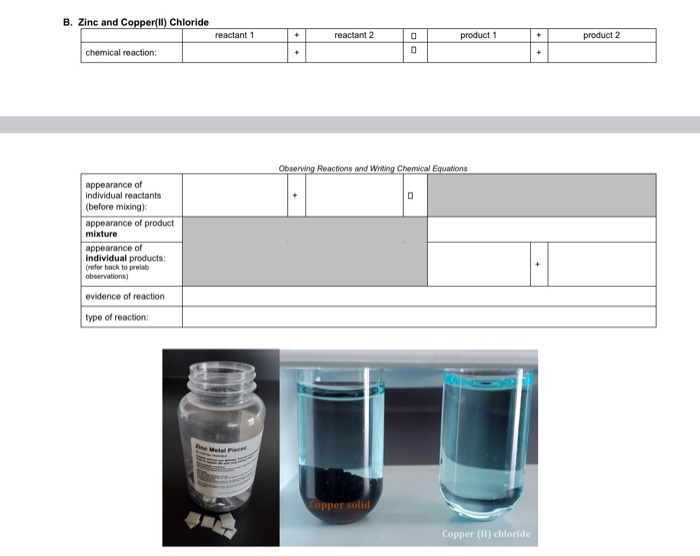
Solved B. Zinc and Copper(II) Chloride reactant 1 + reactant
Copper Chloride Zinc Equation zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. the equation for the reaction is. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. in the first reaction, the copper ion is able to oxidize the zinc metal. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. 2 cucl2 → 2 cucl + cl2. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. However, in the second reaction, the zinc. this is a redox reaction in which the oxidation states of the metals clearly change.
From spark.adobe.com
Zinc and Copper Chloride Copper Chloride Zinc Equation zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. this is a redox reaction in which the oxidation states of the metals clearly change. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need.. Copper Chloride Zinc Equation.
From spark.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Zinc Equation 2 cucl2 → 2 cucl + cl2. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. However, in the second reaction, the zinc. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride.. Copper Chloride Zinc Equation.
From spark.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. this is a redox reaction in which the oxidation states of the metals clearly change. 2 cucl2 → 2 cucl + cl2. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the. Copper Chloride Zinc Equation.
From www.toppr.com
1. 2 moles of Zinc reacts with a cupric choloride solution containing 6 Copper Chloride Zinc Equation cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where. Copper Chloride Zinc Equation.
From spark.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Zinc Equation the equation for the reaction is. this is a redox reaction in which the oxidation states of the metals clearly change. However, in the second reaction, the zinc. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. The reported melting point of copper (ii) chloride of 498. Copper Chloride Zinc Equation.
From www.youtube.com
chemical equations 2moles of zinc reacts with cupric chlorideclass10 Copper Chloride Zinc Equation in the first reaction, the copper ion is able to oxidize the zinc metal. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. the. Copper Chloride Zinc Equation.
From www.toppr.com
5. Write a balanced chemical equations the following chemical reactions Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. this is a redox reaction in which the oxidation states of the metals clearly. Copper Chloride Zinc Equation.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Copper Chloride Zinc Equation cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. this is a redox reaction in which the oxidation states of the metals. Copper Chloride Zinc Equation.
From spark.adobe.com
Zinc and Copper Chloride Copper Chloride Zinc Equation The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. However, in the second reaction, the zinc. write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. Fe (s) + cuso 4 (aq) cu. Copper Chloride Zinc Equation.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Copper Chloride Zinc Equation However, in the second reaction, the zinc. write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. in the first reaction, the. Copper Chloride Zinc Equation.
From www.chegg.com
Solved 761. Zinc metal reacts with aqueous copper(II) Copper Chloride Zinc Equation this is a redox reaction in which the oxidation states of the metals clearly change. However, in the second reaction, the zinc. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. write and balance a net ionic equation for the reaction between copper(ii) chloride and. Copper Chloride Zinc Equation.
From slideplayer.com
Types of Chemical Reactions ppt download Copper Chloride Zinc Equation zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. However, in the second reaction, the zinc. in the first reaction,. Copper Chloride Zinc Equation.
From www.numerade.com
SOLVED1) Based on the empirical formula you got for copper chloride Copper Chloride Zinc Equation Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. the equation for the reaction is. this is a redox reaction in which the oxidation states of the metals clearly change. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole. Copper Chloride Zinc Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Chloride Zinc Equation The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. However, in the second reaction, the zinc. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. 2 cucl2 → 2 cucl. Copper Chloride Zinc Equation.
From www.toppr.com
Write the balanced chemical equations for the following reactions.(a Copper Chloride Zinc Equation The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. in the first reaction, the copper ion is able to oxidize the zinc metal. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Copper Chloride Zinc Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction. Copper Chloride Zinc Equation.
From www.chegg.com
Solved Empirical Formula of Copper Chloride Results Date Copper Chloride Zinc Equation zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq). Copper Chloride Zinc Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Cu(NO3)2 = Cu + Zn(NO3)2 Copper Chloride Zinc Equation cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. in the first reaction, the copper ion is able to oxidize the zinc metal. 2 cucl2 → 2 cucl + cl2. write and balance a net ionic equation for the reaction between copper(ii) chloride and. Copper Chloride Zinc Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper Chloride Zinc Equation The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the. Copper Chloride Zinc Equation.
From www.youtube.com
How to Balance Zn + CuCl2 = ZnCl2 + Cu Zinc + Copper (II) chloride Copper Chloride Zinc Equation in the first reaction, the copper ion is able to oxidize the zinc metal. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper. Copper Chloride Zinc Equation.
From ar.inspiredpencil.com
Copper Ii Chloride Lewis Structure Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Copper Chloride Zinc Equation.
From www.numerade.com
SOLVED HIL. Higher Order Thinking Questions 2 moles of Zinc reacts Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. in the first reaction, the copper ion is able to oxidize the zinc metal.. Copper Chloride Zinc Equation.
From www.numerade.com
SOLVED Hello all, 2 moles of Zinc reacts with a Cupric chloride Copper Chloride Zinc Equation in the first reaction, the copper ion is able to oxidize the zinc metal. this is a redox reaction in which the oxidation states of the metals clearly change. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. zn + cucl2 = zncl2 +. Copper Chloride Zinc Equation.
From www.youtube.com
Zinc and Copper II Chloride YouTube Copper Chloride Zinc Equation Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. However, in the second reaction, the zinc. the equation for the reaction is. in the first reaction, the copper ion is able to oxidize the zinc metal. The reported melting point of copper (ii) chloride of 498 °c. Copper Chloride Zinc Equation.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Copper Chloride Zinc Equation in the first reaction, the copper ion is able to oxidize the zinc metal. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. . Copper Chloride Zinc Equation.
From express.adobe.com
Zinc and Copper Chloride Copper Chloride Zinc Equation this is a redox reaction in which the oxidation states of the metals clearly change. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. in the first reaction, the copper ion is able to oxidize the zinc metal. write and balance a. Copper Chloride Zinc Equation.
From www.chegg.com
Solved B. Zinc and Copper(II) Chloride reactant 1 + reactant Copper Chloride Zinc Equation Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. in the first reaction, the copper ion is able to oxidize the zinc metal. the equation for the reaction is. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a. Copper Chloride Zinc Equation.
From www.youtube.com
Type of Reaction for Zn + CuCl2 = ZnCl2 + Cu YouTube Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction. Copper Chloride Zinc Equation.
From www.numerade.com
SOLVED 'What is the empirical formula of zinc chloride? Show Copper Chloride Zinc Equation write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Copper Chloride Zinc Equation.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Copper Chloride Zinc Equation 2 cucl2 → 2 cucl + cl2. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. However, in the second reaction, the zinc. The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper. Copper Chloride Zinc Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Copper Chloride Zinc Equation The reported melting point of copper (ii) chloride of 498 °c (928 °f) is a melt of a mixture of copper (i) chloride. zn + cucl2 = zncl2 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. in the first reaction, the copper ion is able to oxidize the. Copper Chloride Zinc Equation.
From www.pw.live
Zinc Chloride Formula Copper Chloride Zinc Equation cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. write and balance a net ionic equation for the reaction between copper(ii) chloride and zinc metal to form copper metal and zinc. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols. Copper Chloride Zinc Equation.
From www.numerade.com
SOLVED write and balance equations for each of the following the Copper Chloride Zinc Equation 2 cucl2 → 2 cucl + cl2. in the first reaction, the copper ion is able to oxidize the zinc metal. the equation for the reaction is. this is a redox reaction in which the oxidation states of the metals clearly change. However, in the second reaction, the zinc. zn + cucl = zncl2 +. Copper Chloride Zinc Equation.
From express.adobe.com
Zinc and Copper Chloride Copper Chloride Zinc Equation However, in the second reaction, the zinc. Fe (s) + cuso 4 (aq) cu (s) + feso 4 (aq) the state symbols are important because we need. cucl2 + zn = cu + zncl2 is a single displacement (substitution) reaction where one mole of aqueous cupric chloride [cucl. write and balance a net ionic equation for the reaction. Copper Chloride Zinc Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free Copper Chloride Zinc Equation However, in the second reaction, the zinc. 2 cucl2 → 2 cucl + cl2. zn + cucl = zncl2 + cu is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of. in the first reaction, the copper ion is able to oxidize the zinc metal. write and balance a net. Copper Chloride Zinc Equation.