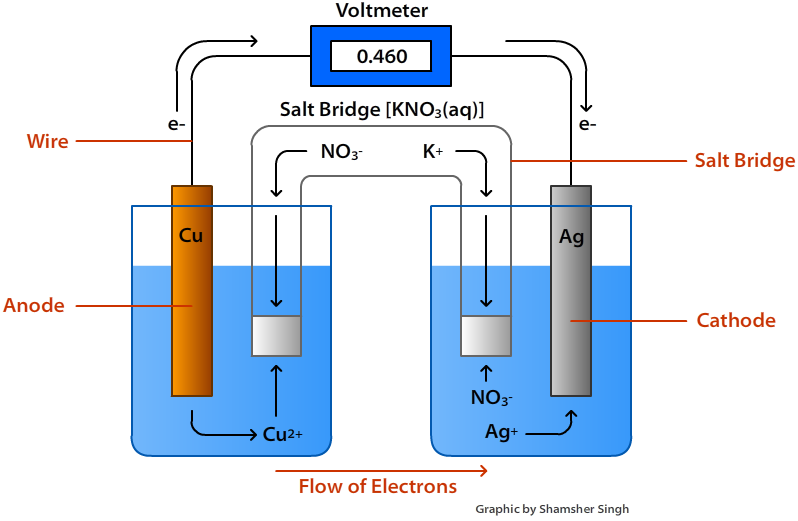
Electrodes In Electrochemical Cell . in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; electrochemical cells have two conductive electrodes, called the anode and the cathode. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. The anode is defined as the electrode where oxidation occurs. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the.
from exoxpbgzu.blob.core.windows.net
an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. electrochemical cells have two conductive electrodes, called the anode and the cathode. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; The anode is defined as the electrode where oxidation occurs.
Define Electrodes In Chemistry Terms at Monte Cordell blog
Electrodes In Electrochemical Cell the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. electrochemical cells have two conductive electrodes, called the anode and the cathode. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. The anode is defined as the electrode where oxidation occurs.
From www.researchgate.net
A threeelectrode electrochemical cell and sample preparation for CV Electrodes In Electrochemical Cell Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. an electrochemical cell. Electrodes In Electrochemical Cell.
From www.researchgate.net
Schematic illustration of threeelectrode electrochemical Hcell Electrodes In Electrochemical Cell the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; The. Electrodes In Electrochemical Cell.
From www.researchgate.net
17. A schematic view of electrochemical setup 3electrode cell in a Electrodes In Electrochemical Cell the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. electrochemical cells have two conductive electrodes, called the anode and the cathode. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. Most electrochemical cells consist of two solutions with metal electrodes. Electrodes In Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Electrodes In Electrochemical Cell in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; electrochemical cells have two conductive electrodes, called the anode and the cathode. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. The anode. Electrodes In Electrochemical Cell.
From mungfali.com
Glass Electrode Diagram Electrodes In Electrochemical Cell Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. The anode is defined as the electrode where oxidation occurs. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. the two compartments of an electrochemical cell where the half reactions. Electrodes In Electrochemical Cell.
From www.researchgate.net
Schematic representation of three electrode electrochemical cell used Electrodes In Electrochemical Cell in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; The anode is defined as the electrode where oxidation occurs. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. the two compartments. Electrodes In Electrochemical Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrodes In Electrochemical Cell The anode is defined as the electrode where oxidation occurs. electrochemical cells have two conductive electrodes, called the anode and the cathode. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how. Electrodes In Electrochemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrodes In Electrochemical Cell Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. electrochemical cells have two conductive electrodes, called the anode and the cathode. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; The anode is defined as the electrode where oxidation occurs. . Electrodes In Electrochemical Cell.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Electrodes In Electrochemical Cell electrochemical cells have two conductive electrodes, called the anode and the cathode. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; an electrochemical cell is a device. Electrodes In Electrochemical Cell.
From www.researchgate.net
The convention three electrode cell configuration for electrochemical Electrodes In Electrochemical Cell Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. The anode is defined as the electrode where oxidation occurs. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. electrochemical cells have two conductive electrodes, called the anode and the cathode.. Electrodes In Electrochemical Cell.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrodes In Electrochemical Cell in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. The anode is defined as the electrode where oxidation occurs. an electrochemical cell is a device capable of either. Electrodes In Electrochemical Cell.
From www.researchgate.net
The electrochemical cell setup. (I) Screen printed electrodes. (II) The Electrodes In Electrochemical Cell in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. . Electrodes In Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrodes In Electrochemical Cell electrochemical cells have two conductive electrodes, called the anode and the cathode. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. an. Electrodes In Electrochemical Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Electrodes In Electrochemical Cell electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as the electrode where oxidation occurs. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; the two compartments of an electrochemical cell where the half reactions occur are called. Electrodes In Electrochemical Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrodes In Electrochemical Cell electrochemical cells have two conductive electrodes, called the anode and the cathode. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in the example of. Electrodes In Electrochemical Cell.
From blog.iorodeo.com
Making a custom electrochemical cell Electrodes In Electrochemical Cell an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. electrochemical cells have two conductive electrodes, called the anode and the cathode. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. in the example of the zn/cu cell we have. Electrodes In Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Electrodes In Electrochemical Cell The anode is defined as the electrode where oxidation occurs. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. the two compartments of an electrochemical cell where. Electrodes In Electrochemical Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrodes In Electrochemical Cell electrochemical cells have two conductive electrodes, called the anode and the cathode. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. an electrochemical cell is a device capable of. Electrodes In Electrochemical Cell.
From www.researchgate.net
Schematic representation of the electrochemical cell used in this Electrodes In Electrochemical Cell In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using. Electrodes In Electrochemical Cell.
From www.researchgate.net
Fourelectrode electrochemical cell. Download Electrodes In Electrochemical Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemical cells have two conductive electrodes, called the anode and the cathode. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. Most electrochemical cells consist of. Electrodes In Electrochemical Cell.
From hinahanap6dschematic.z21.web.core.windows.net
What Forms At The Cathode During Electrolysis Electrodes In Electrochemical Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. In. Electrodes In Electrochemical Cell.
From www.researchgate.net
Diagram of a three electrode electrochemical cell. Download Electrodes In Electrochemical Cell The anode is defined as the electrode where oxidation occurs. electrochemical cells have two conductive electrodes, called the anode and the cathode. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. the two compartments of. Electrodes In Electrochemical Cell.
From www.researchgate.net
Illustration of operation of (a) a threeelectrode electrochemical cell Electrodes In Electrochemical Cell in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. The anode is defined as the electrode where oxidation occurs. Most electrochemical cells consist of. Electrodes In Electrochemical Cell.
From www.metrohm.com
Basic electrochemical cell set up Metrohm Electrodes In Electrochemical Cell an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. The anode is defined as the electrode where oxidation occurs. the two compartments of an electrochemical cell where the half reactions occur. Electrodes In Electrochemical Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrodes In Electrochemical Cell In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell. Electrodes In Electrochemical Cell.
From www.researchgate.net
3 Three electrode electrochemical cell. Electrochemical cells normally Electrodes In Electrochemical Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. The anode is defined as the electrode where oxidation occurs. in the example of the zn/cu cell. Electrodes In Electrochemical Cell.
From www.researchgate.net
Scheme of a classical threeelectrode cell. Download Scientific Diagram Electrodes In Electrochemical Cell the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemical cells have two conductive electrodes, called the anode and the cathode. in the example of the zn/cu cell we have. Electrodes In Electrochemical Cell.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode Electrodes In Electrochemical Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. electrochemical cells have two conductive electrodes, called the anode and the cathode. Most electrochemical cells consist of two. Electrodes In Electrochemical Cell.
From www.researchgate.net
Scheme of an experimental 3 electrode electrochemical cell. Download Electrodes In Electrochemical Cell electrochemical cells have two conductive electrodes, called the anode and the cathode. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. In this tutorial on galvanic. Electrodes In Electrochemical Cell.
From www.researchgate.net
Electrochemical analysis threeelectrode setup. Schematic of the Electrodes In Electrochemical Cell the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in the example of the zn/cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how. Electrodes In Electrochemical Cell.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrodes In Electrochemical Cell Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. an electrochemical. Electrodes In Electrochemical Cell.
From studylib.net
Electrochemical cells Electrodes In Electrochemical Cell an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. The anode is defined as the electrode where oxidation occurs. Most electrochemical cells consist of two solutions with metal. Electrodes In Electrochemical Cell.
From mavink.com
Electrochemical Cell Diagram Electrodes In Electrochemical Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. in the example of the zn/cu cell we. Electrodes In Electrochemical Cell.
From www.researchgate.net
Three electrodes electrochemical cell for testing electrocatalytic Electrodes In Electrochemical Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how. Electrodes In Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes In Electrochemical Cell The anode is defined as the electrode where oxidation occurs. Most electrochemical cells consist of two solutions with metal electrodes and a salt bridge. electrochemical cells have two conductive electrodes, called the anode and the cathode. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. an electrochemical cell. Electrodes In Electrochemical Cell.