Standard Enthalpy Of Formation Mcq . (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. the table shows standard enthalpy changes of formation, ∆f h o. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. (a) always zero (b) always positive (c). a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. enthalpy of formation practice problems. The enthalpy of formation (∆h°f) of an element in its standard state is:
from studylib.net
c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. the table shows standard enthalpy changes of formation, ∆f h o. (a) always zero (b) always positive (c). enthalpy of formation practice problems. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. The enthalpy of formation (∆h°f) of an element in its standard state is:
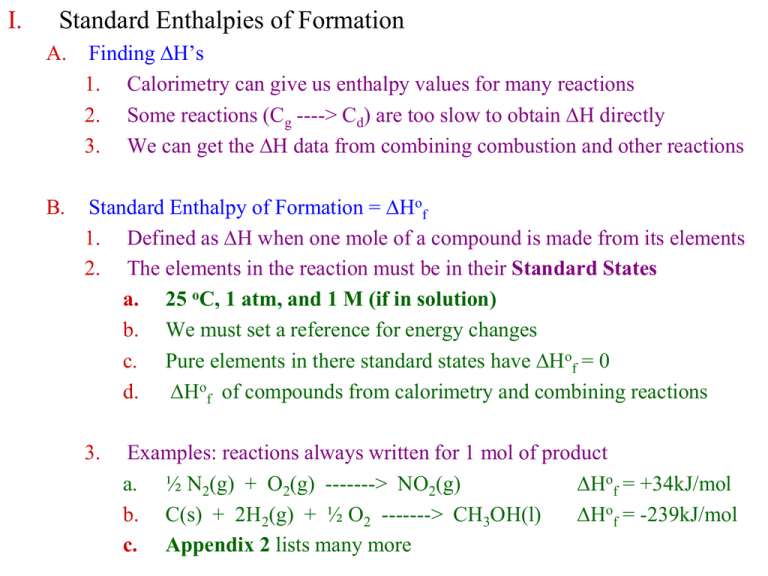
I. Standard Enthalpies of Formation
Standard Enthalpy Of Formation Mcq c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. (a) always zero (b) always positive (c). (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. enthalpy of formation practice problems. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. The enthalpy of formation (∆h°f) of an element in its standard state is: the table shows standard enthalpy changes of formation, ∆f h o.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Mcq (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. enthalpy of formation practice problems. the table shows standard enthalpy changes of formation, ∆f h o. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. The. Standard Enthalpy Of Formation Mcq.
From www.chegg.com
Solved 3.Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Mcq a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c. Standard Enthalpy Of Formation Mcq.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Mcq The enthalpy of formation (∆h°f) of an element in its standard state is: the table shows standard enthalpy changes of formation, ∆f h o. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. a) ∆calculate the standard enthalpy change, ’ $ (), for the. Standard Enthalpy Of Formation Mcq.
From slideplayer.com
Standard Enthalpies of Formation ppt download Standard Enthalpy Of Formation Mcq this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. the table shows standard enthalpy changes of formation, ∆f h o. c a. Standard Enthalpy Of Formation Mcq.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Mcq (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. (a) always zero (b) always positive (c). The enthalpy of formation (∆h°f) of an element. Standard Enthalpy Of Formation Mcq.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Mcq this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. (a) always zero (b) always positive (c). this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented. Standard Enthalpy Of Formation Mcq.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Mcq the table shows standard enthalpy changes of formation, ∆f h o. The enthalpy of formation (∆h°f) of an element in its standard state is: (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. c a c o 3 decomposes according to the balanced equation. Standard Enthalpy Of Formation Mcq.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Mcq enthalpy of formation practice problems. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is. Standard Enthalpy Of Formation Mcq.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Mcq enthalpy of formation practice problems. the table shows standard enthalpy changes of formation, ∆f h o. (a) always zero (b) always positive (c). c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided. Standard Enthalpy Of Formation Mcq.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Mcq (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. The enthalpy of formation (∆h°f) of an element in its standard state is: a) ∆calculate the standard enthalpy change,. Standard Enthalpy Of Formation Mcq.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Mcq (a) always zero (b) always positive (c). enthalpy of formation practice problems. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) +. Standard Enthalpy Of Formation Mcq.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Mcq (a) always zero (b) always positive (c). The enthalpy of formation (∆h°f) of an element in its standard state is: this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. (b). Standard Enthalpy Of Formation Mcq.
From quizzlistreplevies.z13.web.core.windows.net
Heats Of Formation Equation Standard Enthalpy Of Formation Mcq this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. the table shows standard enthalpy changes of formation, ∆f h o. c a c o 3 decomposes according to the balanced equation c. Standard Enthalpy Of Formation Mcq.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Mcq (a) always zero (b) always positive (c). The enthalpy of formation (∆h°f) of an element in its standard state is: enthalpy of formation practice problems. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation. Standard Enthalpy Of Formation Mcq.
From www.iitianacademy.com
AP Chemistry 6.8 Enthalpy of Formation Exam Style questions with Standard Enthalpy Of Formation Mcq enthalpy of formation practice problems. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in. Standard Enthalpy Of Formation Mcq.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Mcq this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. The enthalpy of formation (∆h°f) of an element in its standard state is: (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. c. Standard Enthalpy Of Formation Mcq.
From www.studocu.com
Enthalpy definitions The Standard Enthalpy of Formation, H f, 298, is Standard Enthalpy Of Formation Mcq (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. (a) always zero (b) always positive (c). the table shows standard enthalpy changes of formation, ∆f h o. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of. Standard Enthalpy Of Formation Mcq.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Mcq this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. c a. Standard Enthalpy Of Formation Mcq.
From studylib.net
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy Standard Enthalpy Of Formation Mcq this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. . Standard Enthalpy Of Formation Mcq.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6319219 Standard Enthalpy Of Formation Mcq (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. enthalpy of formation practice problems. a) ∆calculate the standard enthalpy change, ’ $. Standard Enthalpy Of Formation Mcq.
From www.chegg.com
NCho 1996 22. The standard enthalpy of formation ( Standard Enthalpy Of Formation Mcq c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. enthalpy of formation practice problems. this online quiz is intended to give you extra practice in enthalpy calculations for a variety. Standard Enthalpy Of Formation Mcq.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Mcq The enthalpy of formation (∆h°f) of an element in its standard state is: this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. enthalpy of formation practice problems. the table shows standard enthalpy changes of formation, ∆f h o. this online quiz is intended to give you extra practice in. Standard Enthalpy Of Formation Mcq.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Mcq this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. the table shows standard enthalpy changes of formation, ∆f h o. enthalpy of formation practice problems. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c. Standard Enthalpy Of Formation Mcq.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Mcq The enthalpy of formation (∆h°f) of an element in its standard state is: this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. (a) always zero (b) always positive (c). (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known. Standard Enthalpy Of Formation Mcq.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Mcq this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. enthalpy of formation practice problems. The enthalpy of formation (∆h°f) of an element in its standard state is: this online quiz is intended. Standard Enthalpy Of Formation Mcq.
From www.chegg.com
Solved 8) a) Define "standard enthalpy change of formation." Standard Enthalpy Of Formation Mcq (a) always zero (b) always positive (c). The enthalpy of formation (∆h°f) of an element in its standard state is: this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s). Standard Enthalpy Of Formation Mcq.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Mcq this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. The enthalpy of formation (∆h°f) of an element in its standard state is: the table shows standard enthalpy changes of formation, ∆f h o. (a) always zero (b) always positive (c). c a c o 3 decomposes according to the balanced. Standard Enthalpy Of Formation Mcq.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Mcq c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation.. Standard Enthalpy Of Formation Mcq.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Mcq enthalpy of formation practice problems. c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation. Standard Enthalpy Of Formation Mcq.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Mcq c a c o 3 decomposes according to the balanced equation c a c o 3 (s) → c a o (s) + c o 2 (g).based on the standard enthalpies of formation provided in the. The enthalpy of formation (∆h°f) of an element in its standard state is: (b) the change in enthalpy when 1 mole of the. Standard Enthalpy Of Formation Mcq.
From www.youtube.com
Standard Enthalpy of Formation YouTube Standard Enthalpy Of Formation Mcq The enthalpy of formation (∆h°f) of an element in its standard state is: this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. enthalpy of formation practice problems. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. (b) the. Standard Enthalpy Of Formation Mcq.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Mcq this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. (b) the change in enthalpy when 1 mole of the compound is formed under standard conditions is known as standard enthalpy of formation. enthalpy of formation practice problems. a) ∆calculate the standard enthalpy change, ’ $. Standard Enthalpy Of Formation Mcq.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Mcq the table shows standard enthalpy changes of formation, ∆f h o. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. (a) always zero (b) always positive (c). enthalpy. Standard Enthalpy Of Formation Mcq.
From www.scribd.com
Standard Enthalpy of Formation PDF Solvation Chemical Process Standard Enthalpy Of Formation Mcq (a) always zero (b) always positive (c). a) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. this set of basic chemical engineering multiple choice questions & answers (mcqs) focuses on “standard heat. this online quiz is intended to give you extra practice in enthalpy calculations for a variety of. Standard Enthalpy Of Formation Mcq.
From blogs.glowscotland.org.uk
Enthalpy Change MCQs CfE Higher Chemistry Revision Questions Standard Enthalpy Of Formation Mcq The enthalpy of formation (∆h°f) of an element in its standard state is: this online quiz is intended to give you extra practice in enthalpy calculations for a variety of physical and chemical changes. (a) always zero (b) always positive (c). c a c o 3 decomposes according to the balanced equation c a c o 3 (s). Standard Enthalpy Of Formation Mcq.