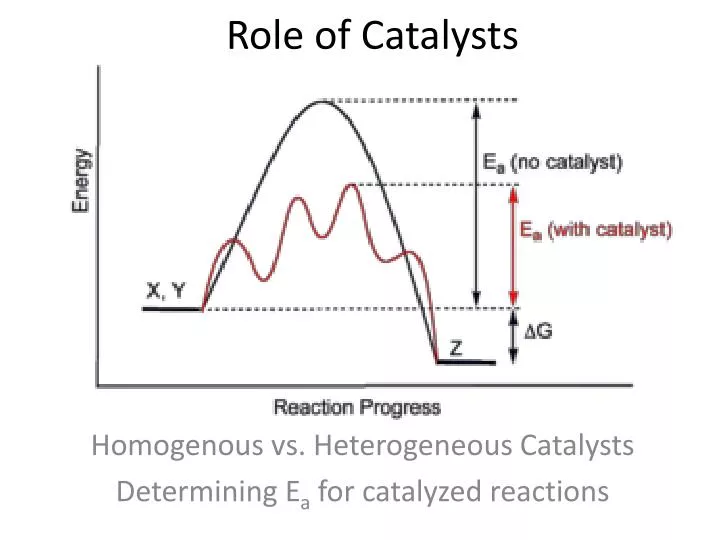
How Does A Catalyst Work Quizlet . a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. A + b ® c. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. how catalysts work. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: the catalyst provides a different reaction path with a lower activation energy. how does a catalyst work? study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity.
from www.slideserve.com
a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: the catalyst provides a different reaction path with a lower activation energy. how does a catalyst work? study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. A + b ® c. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. how catalysts work.
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169
How Does A Catalyst Work Quizlet a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. the catalyst provides a different reaction path with a lower activation energy. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: A + b ® c. how does a catalyst work? Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. how catalysts work.
From azurechemical.com
How Does a Selective Catalytic Reduction System Work? How Does A Catalyst Work Quizlet a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants.. How Does A Catalyst Work Quizlet.
From dxxaaiodeco.blob.core.windows.net
Catalyst Chemical Formula at Paul Lowther blog How Does A Catalyst Work Quizlet a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. how does a. How Does A Catalyst Work Quizlet.
From idealcalibrations.com
How Do LEL Catalytic/Pellister Bead Sensors Work? Ideal Calibrations How Does A Catalyst Work Quizlet study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a student designs an experiment. How Does A Catalyst Work Quizlet.
From www.youtube.com
What Does A Catalyst Do? YouTube How Does A Catalyst Work Quizlet a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. . How Does A Catalyst Work Quizlet.
From exogzbwud.blob.core.windows.net
Enzymes Contain Hydrolytic at Dennis Hernandez blog How Does A Catalyst Work Quizlet Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. A. How Does A Catalyst Work Quizlet.
From exovfwjzd.blob.core.windows.net
Catalysts Speed Up Chemical Reactions True Or False at Paul Bernal blog How Does A Catalyst Work Quizlet A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: how. How Does A Catalyst Work Quizlet.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 How Does A Catalyst Work Quizlet study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. A + b ® c. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: study with quizlet and memorize flashcards containing terms like catalysed reaction,. How Does A Catalyst Work Quizlet.
From www.hanlin.com
IB DP Chemistry HL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 How Does A Catalyst Work Quizlet how catalysts work. A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. the catalyst provides a different reaction path with a lower activation energy. A + b ® c.. How Does A Catalyst Work Quizlet.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of How Does A Catalyst Work Quizlet the catalyst provides a different reaction path with a lower activation energy. A + b ® c. A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: Catalysts. How Does A Catalyst Work Quizlet.
From dzasnqwfeco.blob.core.windows.net
Can You Put Any Catalytic Converter On Any Car at Jerry Goodnight blog How Does A Catalyst Work Quizlet a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: A + b ® c. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. How Does A Catalyst Work Quizlet.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog How Does A Catalyst Work Quizlet the catalyst provides a different reaction path with a lower activation energy. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. a student designs an experiment to test substances x,. How Does A Catalyst Work Quizlet.
From thendbcatalyst.com
‘Quizlet’ moves many popular features to payment plan The Catalyst How Does A Catalyst Work Quizlet A + b ® c. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: A catalyst works by providing a convenient surface that enables a different route for a chemical reaction. How Does A Catalyst Work Quizlet.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Does A Catalyst Work Quizlet A + b ® c. study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a catalyst works by changing the specific way in which the reaction occurs, called its. How Does A Catalyst Work Quizlet.
From cemzdbvf.blob.core.windows.net
Enzymes Function As A Catalyst By Quizlet at Courtney Mason blog How Does A Catalyst Work Quizlet study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. how does a catalyst work? a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. the catalyst provides a different reaction path with a lower activation energy. a student designs an experiment. How Does A Catalyst Work Quizlet.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram How Does A Catalyst Work Quizlet a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. the catalyst provides a different reaction path with a lower activation energy. how does a catalyst work? a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for. How Does A Catalyst Work Quizlet.
From ceaevwdd.blob.core.windows.net
Do Catalytic Converters Work When Cold at Veda Larue blog How Does A Catalyst Work Quizlet a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. A catalyst works by providing a convenient surface that. How Does A Catalyst Work Quizlet.
From www.vedantu.com
Birch Reduction and Lindlar Catalyst Important Concepts and Tips for JEE How Does A Catalyst Work Quizlet a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. how does a catalyst work? A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to.. How Does A Catalyst Work Quizlet.
From www.slideshare.net
Biology 2.4 How Does A Catalyst Work Quizlet how catalysts work. the catalyst provides a different reaction path with a lower activation energy. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the.. How Does A Catalyst Work Quizlet.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube How Does A Catalyst Work Quizlet the catalyst provides a different reaction path with a lower activation energy. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. study with quizlet and memorize flashcards containing terms. How Does A Catalyst Work Quizlet.
From cekhunhy.blob.core.windows.net
What Is The Function Of A Catalyst Quizlet at Denise West blog How Does A Catalyst Work Quizlet a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up. How Does A Catalyst Work Quizlet.
From guidelibdiapedetic.z22.web.core.windows.net
Catalyst Diagram Chemistry How Does A Catalyst Work Quizlet the catalyst provides a different reaction path with a lower activation energy. how catalysts work. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a student designs an experiment to. How Does A Catalyst Work Quizlet.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub How Does A Catalyst Work Quizlet study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. A + b ® c. Catalysts permit. How Does A Catalyst Work Quizlet.
From quizlet.com
Biology 2.22.3 Flashcards Quizlet How Does A Catalyst Work Quizlet how catalysts work. how does a catalyst work? Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. A + b ® c. a catalyst works by changing. How Does A Catalyst Work Quizlet.
From www.youtube.com
How does a CATALYST work ? YouTube How Does A Catalyst Work Quizlet study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. how catalysts work. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition.. How Does A Catalyst Work Quizlet.
From www.tffn.net
How Does Catalyst Work? A Comprehensive Guide to Understanding How Does A Catalyst Work Quizlet a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. how does a catalyst work? how catalysts work. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular. How Does A Catalyst Work Quizlet.
From guidefixcasiranilv.z4.web.core.windows.net
How To Read Energy Diagrams Chemistry How Does A Catalyst Work Quizlet A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. A + b ® c. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. the catalyst provides a different reaction path with a lower activation energy. study with quizlet. How Does A Catalyst Work Quizlet.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis How Does A Catalyst Work Quizlet A catalyst works by providing a convenient surface that enables a different route for a chemical reaction to. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. study with. How Does A Catalyst Work Quizlet.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation How Does A Catalyst Work Quizlet A + b ® c. the catalyst provides a different reaction path with a lower activation energy. how catalysts work. how does a catalyst work? Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a catalyst works by changing the specific way in which the reaction. How Does A Catalyst Work Quizlet.
From cetavdrz.blob.core.windows.net
Lipases Proteases And Amylases Are Quizlet at Hazel Wallace blog How Does A Catalyst Work Quizlet how does a catalyst work? a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. a catalyst works by changing the specific way in which. How Does A Catalyst Work Quizlet.
From cenkdqaq.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Certain Reaction At 27 at How Does A Catalyst Work Quizlet A + b ® c. how catalysts work. how does a catalyst work? a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. the catalyst provides a different reaction path with a lower activation energy.. How Does A Catalyst Work Quizlet.
From www.researchgate.net
Schematic illustrations of heterogeneous catalyst systems of Cu NPs on How Does A Catalyst Work Quizlet the catalyst provides a different reaction path with a lower activation energy. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process, selectivity. A + b ® c. A catalyst works by providing a convenient surface. How Does A Catalyst Work Quizlet.
From usermanualcarrycot.z21.web.core.windows.net
How To Identify Catalytic Converter How Does A Catalyst Work Quizlet A + b ® c. the catalyst provides a different reaction path with a lower activation energy. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. a catalyst works by changing the specific way in which the reaction occurs, called its mechanism. how does a catalyst work?. How Does A Catalyst Work Quizlet.
From ar.inspiredpencil.com
Catalyst Examples For Kids How Does A Catalyst Work Quizlet a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. how catalysts work. study with quizlet and memorize flashcards containing terms like how does catalyst work in speeding up a reaction?, the. a catalyst works by changing the specific way in which the reaction occurs, called its. How Does A Catalyst Work Quizlet.
From dpzvtzafeco.blob.core.windows.net
Exhaust Catalytic Converter Location at Cheryl Tran blog How Does A Catalyst Work Quizlet how does a catalyst work? a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. the catalyst provides a different reaction path with a lower activation energy. A +. How Does A Catalyst Work Quizlet.
From ceygheqo.blob.core.windows.net
Catalytic Converter Working Principle Pdf at Sylvia Duarte blog How Does A Catalyst Work Quizlet how catalysts work. a student designs an experiment to test substances x, y, and z, to determine which one is a catalyst for the reaction: Catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition. study with quizlet and memorize flashcards containing terms like catalysed reaction, unimolecular process,. How Does A Catalyst Work Quizlet.