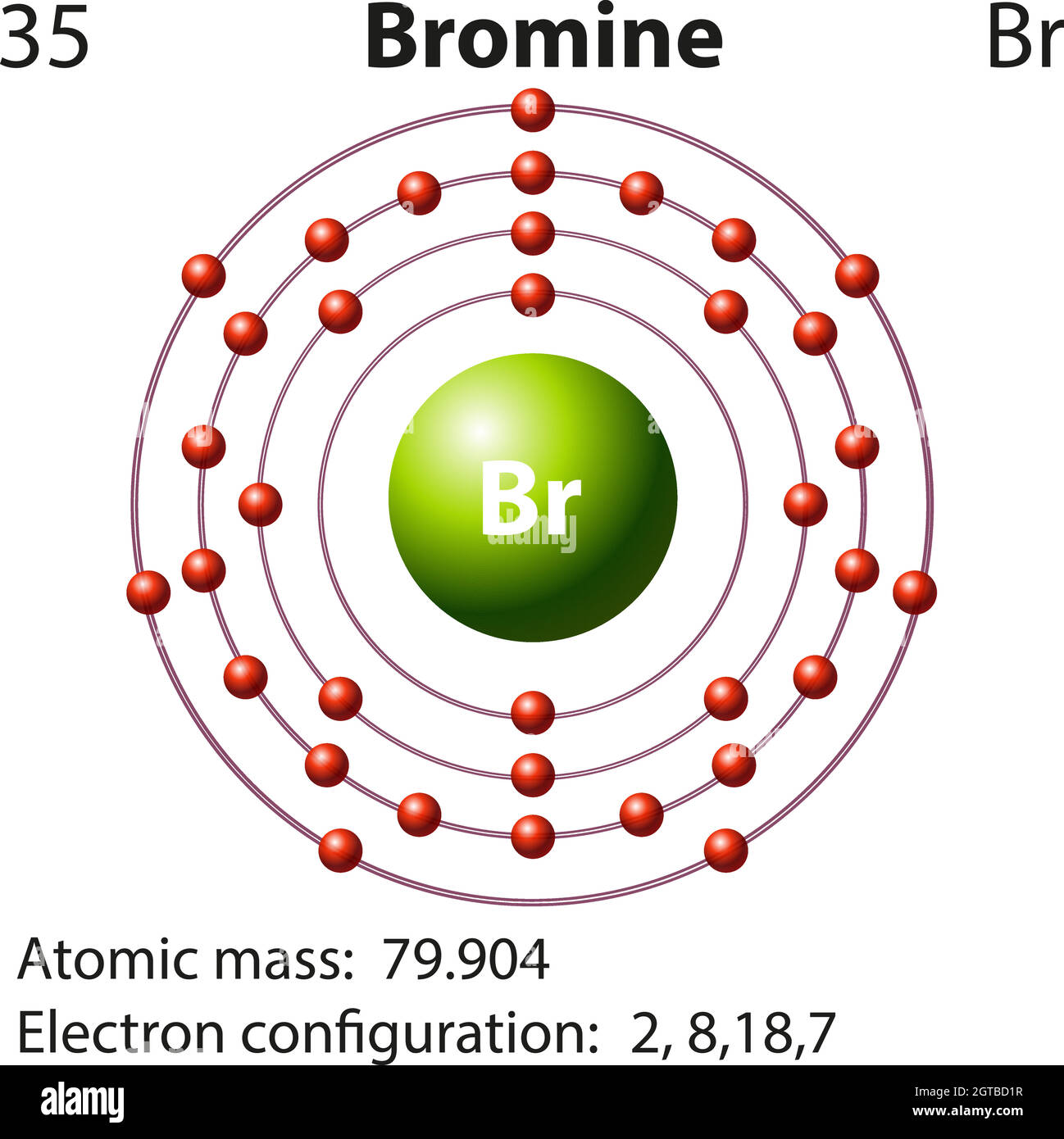
Bromine One Electron . The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. It has an atomic weight of 79.904 and a mass. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. On the pauling scale, bromine’s electronegativity is 2.96. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was the first element to be extracted.
from klaehyagl.blob.core.windows.net
It was the first element to be extracted. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. It has an atomic weight of 79.904 and a mass. On the pauling scale, bromine’s electronegativity is 2.96. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine’s electron configuration is [ar] 3d10 4s2 4p5.
Bromine Molecule Formula at Antonio Godines blog
Bromine One Electron It was the first element to be extracted. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. On the pauling scale, bromine’s electronegativity is 2.96. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. It was the first element to be extracted. It has an atomic weight of 79.904 and a mass. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine One Electron The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. Bromine is the 35th element in the periodic table. Bromine One Electron.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine One Electron It has an atomic weight of 79.904 and a mass. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine has 7 valence electrons,. Bromine One Electron.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine One Electron The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. On the pauling scale, bromine’s electronegativity is 2.96. It has an atomic weight of 79.904 and a mass. It was the first element to be extracted. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive. Bromine One Electron.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Bromine One Electron This can be shortened to #[ar] 4s^2 3d^10 4p^5#. It has an atomic weight of 79.904 and a mass. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. The bromine electron. Bromine One Electron.
From www.alamy.com
Bromine element hires stock photography and images Alamy Bromine One Electron The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. In this article today we are going to tell. Bromine One Electron.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine One Electron Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. It has an atomic weight of 79.904 and a mass. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise. Bromine One Electron.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine One Electron In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. It has an atomic weight of 79.904 and a mass. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. Bromine has 7 valence electrons,. Bromine One Electron.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine One Electron It has an atomic weight of 79.904 and a mass. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. It was the first element to be extracted. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. Bromine has 7 valence. Bromine One Electron.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube Bromine One Electron This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. On the pauling scale, bromine’s electronegativity is 2.96. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. It has. Bromine One Electron.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. On the pauling scale, bromine’s electronegativity is 2.96. Bromine is the 35th element in the periodic table and has a symbol of br. Bromine One Electron.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า Bromine One Electron The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was the first element to be extracted. The bromine electron configuration, denoted as [ar]. Bromine One Electron.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine One Electron The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. It was the first element to be extracted. The bromine electron configuration, denoted as. Bromine One Electron.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine One Electron This can be shortened to #[ar] 4s^2 3d^10 4p^5#. It was the first element to be extracted. It has an atomic weight of 79.904 and a mass. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has 7 valence electrons,. Bromine One Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine One Electron It has an atomic weight of 79.904 and a mass. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. On the pauling scale, bromine’s electronegativity is 2.96. The. Bromine One Electron.
From ceojpsva.blob.core.windows.net
Bromine Electron Dot Notation at Alana Jager blog Bromine One Electron In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. It was the first element to be extracted. On the pauling scale, bromine’s electronegativity is 2.96. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to. Bromine One Electron.
From klaehyagl.blob.core.windows.net
Bromine Molecule Formula at Antonio Godines blog Bromine One Electron It has an atomic weight of 79.904 and a mass. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement. Bromine One Electron.
From www.dreamstime.com
Bromine stock illustration. Illustration of render, formula 139651101 Bromine One Electron The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. On the pauling scale, bromine’s electronegativity is 2.96. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine has. Bromine One Electron.
From www.alamy.com
Bromine atom, with mass and energy levels. Vector illustration Stock Bromine One Electron Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. It was the first element to be extracted. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or. Bromine One Electron.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Bromine One Electron This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement. Bromine One Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine One Electron The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. It has an atomic weight of 79.904 and a mass. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. The electron configuration of bromine refers to the arrangement of electrons. Bromine One Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine One Electron The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number. Bromine One Electron.
From fity.club
Electron Configuration Of Bromine Bromine One Electron Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. It was the first element to be extracted. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or. Bromine One Electron.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. It was the first element to be extracted. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine. Bromine One Electron.
From mungfali.com
Bromine Electron Dot Diagram Bromine One Electron It has an atomic weight of 79.904 and a mass. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. On the pauling scale, bromine’s electronegativity is 2.96. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p. Bromine One Electron.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Bromine is the 35th element in the. Bromine One Electron.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Bromine One Electron In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. It has an atomic weight of 79.904 and a mass. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as. Bromine One Electron.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. This can be shortened to #[ar] 4s^2. Bromine One Electron.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. It was the first element to be extracted. In this article today we are going to tell you about the electron configuration of. Bromine One Electron.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine One Electron The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. It has an atomic weight of 79.904 and a. Bromine One Electron.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine One Electron This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine’s electron configuration is [ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. The electron configuration of bromine refers. Bromine One Electron.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine One Electron Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6. Bromine One Electron.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine One Electron In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine is. Bromine One Electron.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine One Electron On the pauling scale, bromine’s electronegativity is 2.96. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. It has an atomic weight of 79.904 and a mass. The electron configuration of bromine. Bromine One Electron.
From utedzz.blogspot.com
Bromine Periodic Table Square Periodic Table Timeline Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. On the pauling scale, bromine’s electronegativity is 2.96. It has an atomic weight of 79.904 and a mass. Bromine has 7 valence electrons,. Bromine One Electron.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine One Electron The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. It has an atomic weight of. Bromine One Electron.