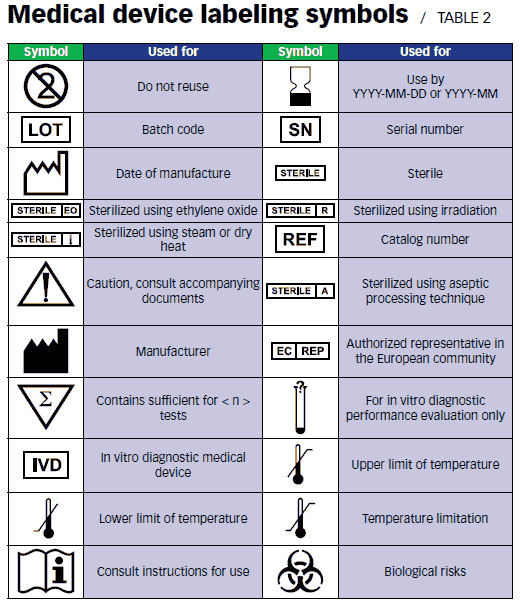
Fda Private Label Distributor Medical Device . fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended.
from www.vrogue.co
more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device.
Fda Medical Device Label Symbols vrogue.co
Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended.
From www.vrogue.co
Nicelabel Blog Medical Device Labeling Compliance Cha vrogue.co Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. and a “private label. Fda Private Label Distributor Medical Device.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. learn about. Fda Private Label Distributor Medical Device.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. fda. Fda Private Label Distributor Medical Device.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes. Fda Private Label Distributor Medical Device.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. fda. Fda Private Label Distributor Medical Device.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Fda Private Label Distributor Medical Device fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device.. Fda Private Label Distributor Medical Device.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. . Fda Private Label Distributor Medical Device.
From dxodfmusp.blob.core.windows.net
Medical Device Packaging Labels at Mary Backstrom blog Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. fda. Fda Private Label Distributor Medical Device.
From www.presentationeze.com
Medical Device Regulation MDR 2017 745 PresentationEZE Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. . Fda Private Label Distributor Medical Device.
From exoptcoyn.blob.core.windows.net
Medical Device Label Font Size at Loren Pierce blog Fda Private Label Distributor Medical Device learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. and a. Fda Private Label Distributor Medical Device.
From medicaldeviceacademy.com
Private Labeled Devices with FDA Approval Medical Device Academy Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. a firm that does. Fda Private Label Distributor Medical Device.
From andamanmed.com
Medical device labeling requirements in the Philippines Fda Private Label Distributor Medical Device learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. more specifically,. Fda Private Label Distributor Medical Device.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Fda Private Label Distributor Medical Device learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. more specifically, fda calls. Fda Private Label Distributor Medical Device.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Fda Private Label Distributor Medical Device fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. learn about. Fda Private Label Distributor Medical Device.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor.. Fda Private Label Distributor Medical Device.
From www.youtube.com
Introduction to Medical Device Labeling Symbols YouTube Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. fda. Fda Private Label Distributor Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. and. Fda Private Label Distributor Medical Device.
From www.pinterest.jp
Épinglé sur Pharmaceutical Labeling Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. a firm that does. Fda Private Label Distributor Medical Device.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Fda Private Label Distributor Medical Device learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm. Fda Private Label Distributor Medical Device.
From decomplix.com
Which MDR requirements apply to distributors of medical devices? Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. and. Fda Private Label Distributor Medical Device.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm. Fda Private Label Distributor Medical Device.
From old.sermitsiaq.ag
Medical Device Label Template Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended.. Fda Private Label Distributor Medical Device.
From texaslabelprinters.com
Medical Device Label Printers UDI Label Printers Fda Private Label Distributor Medical Device fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. more specifically,. Fda Private Label Distributor Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Private Label Distributor Medical Device fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. . Fda Private Label Distributor Medical Device.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. fda has. Fda Private Label Distributor Medical Device.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Private Label Distributor Medical Device learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. a firm. Fda Private Label Distributor Medical Device.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Private Label Distributor Medical Device and a “private label distributor” is an establishment that exports a device manufactured and owned by another party,. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. a firm that does. Fda Private Label Distributor Medical Device.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended.. Fda Private Label Distributor Medical Device.
From www.scribd.com
FDA Requirements for Medical Devices Medical Device Authentication Fda Private Label Distributor Medical Device learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its.. Fda Private Label Distributor Medical Device.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended.. Fda Private Label Distributor Medical Device.
From acf.com.tr
OEM PLM under MDR. Which model you will choose? Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as. Fda Private Label Distributor Medical Device.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Private Label Distributor Medical Device a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. fda. Fda Private Label Distributor Medical Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Private Label Distributor Medical Device learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its.. Fda Private Label Distributor Medical Device.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Fda Private Label Distributor Medical Device fda has stated that private label distributors are labelers for udi purposes, but has not provided a rationale or citation. more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes. Fda Private Label Distributor Medical Device.
From paragondsi.com
UDI Unique Device Identification for Single and Multiple Uses Fda Private Label Distributor Medical Device more specifically, fda calls such a distributor a “private label distributor” and officially defines that as a distributor. a firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its. learn about the minimum labeling requirements for all medical devices, such as name and place of business, intended.. Fda Private Label Distributor Medical Device.