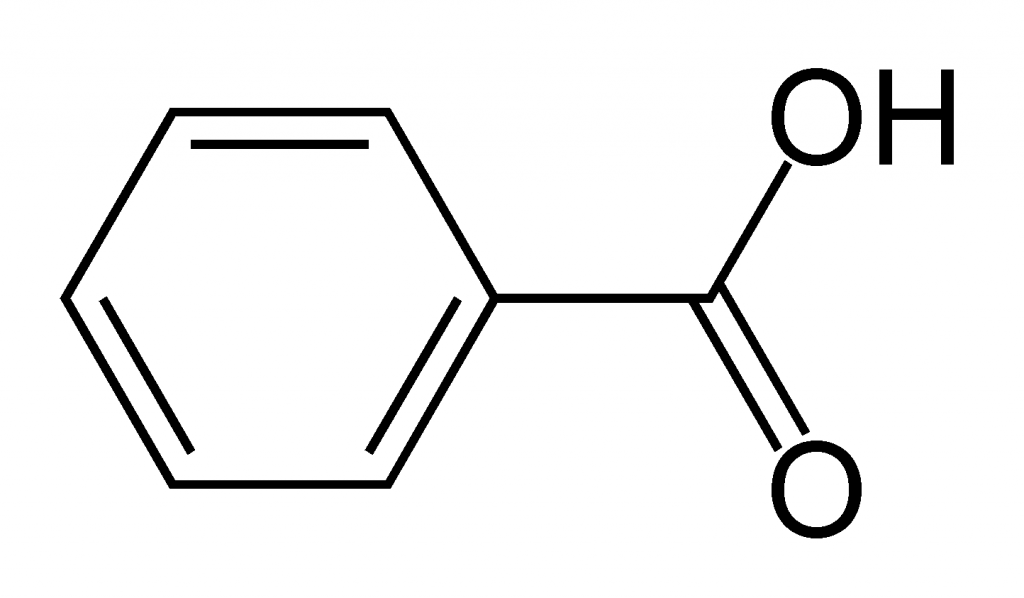
Vacuum Distillation Benzoic Acid . Add approximately 0.5 g (record the actual mass to four decimal places) of the. benzoic acid is not very soluble in cold water, but it is soluble in hot water. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — vacuum distillation was operated under the following conditions: Obtain a sample of benzoic acid. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. The purpose of this experiment is to learn the. Top temperature at 186°c, top pressure of 16.
from www.writework.com
— the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. Obtain a sample of benzoic acid. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Top temperature at 186°c, top pressure of 16. The purpose of this experiment is to learn the. — vacuum distillation was operated under the following conditions: Add approximately 0.5 g (record the actual mass to four decimal places) of the. benzoic acid is not very soluble in cold water, but it is soluble in hot water. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to.
recrystallization of Benzoic acid WriteWork
Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Add approximately 0.5 g (record the actual mass to four decimal places) of the. Top temperature at 186°c, top pressure of 16. benzoic acid is not very soluble in cold water, but it is soluble in hot water. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. The purpose of this experiment is to learn the. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — vacuum distillation was operated under the following conditions: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Obtain a sample of benzoic acid.
From www.scribd.com
Benzoic Acid PDF Distillation Acid Vacuum Distillation Benzoic Acid benzoic acid is not very soluble in cold water, but it is soluble in hot water. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. The purpose of. Vacuum Distillation Benzoic Acid.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: Add. Vacuum Distillation Benzoic Acid.
From elitetradebd.com
Vacuum Distillation Glass Elite Scientific & Meditech Co. Vacuum Distillation Benzoic Acid The purpose of this experiment is to learn the. Obtain a sample of benzoic acid. — vacuum distillation was operated under the following conditions: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. this product was distilled under vacuum to separate a benzoic acid fraction and a. Vacuum Distillation Benzoic Acid.
From www.slideshare.net
Experiment 4 purification recrystallization of benzoic acid Vacuum Distillation Benzoic Acid this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. benzoic acid is not very soluble in cold water, but it is soluble in hot water. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: — vacuum distillation. Vacuum Distillation Benzoic Acid.
From www.researchgate.net
Scheme 2 Synthesis of ebselen from benzoic acid by ortholithiation of Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Top temperature at 186°c, top pressure of 16. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. this product was distilled under vacuum to separate a benzoic acid. Vacuum Distillation Benzoic Acid.
From www.toppr.com
Benzoic acid reacts with Ca (OH)2 . The product obtained on dry Vacuum Distillation Benzoic Acid The purpose of this experiment is to learn the. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Obtain a sample of benzoic acid. — vacuum distillation was operated under the following conditions: — the mixture to be separated is a solution in methylene chloride of three. Vacuum Distillation Benzoic Acid.
From wastechengineering.com
Vacuum Distillation Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. The purpose of this experiment is to learn the. Add approximately 0.5 g (record the actual mass to four decimal places) of the. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was. Vacuum Distillation Benzoic Acid.
From www.youtube.com
Making Benzoic Acid (from sodium benzoate) YouTube Vacuum Distillation Benzoic Acid Add approximately 0.5 g (record the actual mass to four decimal places) of the. Top temperature at 186°c, top pressure of 16. benzoic acid is not very soluble in cold water, but it is soluble in hot water. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. . Vacuum Distillation Benzoic Acid.
From www.vacuumfiltrations.com
Main Steps of Vacuum Filtration Hawach Scientific Co., Ltd Vacuum Distillation Benzoic Acid benzoic acid is not very soluble in cold water, but it is soluble in hot water. Obtain a sample of benzoic acid. Top temperature at 186°c, top pressure of 16. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. The purpose of this experiment is to learn. Vacuum Distillation Benzoic Acid.
From www.youtube.com
Purification of Benzoic Acid by re crystallization from water YouTube Vacuum Distillation Benzoic Acid Add approximately 0.5 g (record the actual mass to four decimal places) of the. Top temperature at 186°c, top pressure of 16. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — the mixture to be separated is a solution in methylene chloride of three different aromatic. Vacuum Distillation Benzoic Acid.
From www.wikidoc.org
Benzoic acid wikidoc Vacuum Distillation Benzoic Acid Top temperature at 186°c, top pressure of 16. Add approximately 0.5 g (record the actual mass to four decimal places) of the. The purpose of this experiment is to learn the. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — the mixture to be separated is a. Vacuum Distillation Benzoic Acid.
From www.researchgate.net
Schematic of reflux setup. Download Scientific Diagram Vacuum Distillation Benzoic Acid Add approximately 0.5 g (record the actual mass to four decimal places) of the. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. Top temperature at 186°c, top pressure of 16. — vacuum distillation was operated under the following conditions: — to recycle the resources and. Vacuum Distillation Benzoic Acid.
From www.slideshare.net
Experiment 4 purification recrystallization of benzoic acid Vacuum Distillation Benzoic Acid — vacuum distillation was operated under the following conditions: The purpose of this experiment is to learn the. Obtain a sample of benzoic acid. Add approximately 0.5 g (record the actual mass to four decimal places) of the. Top temperature at 186°c, top pressure of 16. benzoic acid is not very soluble in cold water, but it is. Vacuum Distillation Benzoic Acid.
From classnotes.org.in
Purification of Organic Compounds Chemistry, Class 11, Organic Vacuum Distillation Benzoic Acid this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. Obtain a sample of benzoic acid. benzoic acid is not very soluble in cold water, but it is soluble in hot water. — the mixture to be separated is a solution in methylene chloride of three different. Vacuum Distillation Benzoic Acid.
From www.coursehero.com
[Solved] Show the complete reaction mechanism for the synthesis of Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: The purpose of this experiment is to learn the. — vacuum distillation was operated under the following conditions: Top temperature at. Vacuum Distillation Benzoic Acid.
From www.slideshare.net
Experiment 4 purification recrystallization of benzoic acid Vacuum Distillation Benzoic Acid The purpose of this experiment is to learn the. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Obtain a sample of benzoic acid. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — the mixture to. Vacuum Distillation Benzoic Acid.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Vacuum Distillation Benzoic Acid Add approximately 0.5 g (record the actual mass to four decimal places) of the. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — to recycle the resources and. Vacuum Distillation Benzoic Acid.
From www.warlimedia.com
Vacuum Distillation Apparatus Vacuum Distillation Benzoic Acid Add approximately 0.5 g (record the actual mass to four decimal places) of the. benzoic acid is not very soluble in cold water, but it is soluble in hot water. Top temperature at 186°c, top pressure of 16. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst.. Vacuum Distillation Benzoic Acid.
From studylib.net
Experimental Synthesis of benzyl alcohol and benzoic acid Vacuum Distillation Benzoic Acid benzoic acid is not very soluble in cold water, but it is soluble in hot water. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. —. Vacuum Distillation Benzoic Acid.
From www.youtube.com
Vacuum Distillation YouTube Vacuum Distillation Benzoic Acid The purpose of this experiment is to learn the. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: — vacuum distillation was operated under the following conditions: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Obtain a sample. Vacuum Distillation Benzoic Acid.
From www.researchgate.net
Diagram of the vacuum distillation still used for purification Vacuum Distillation Benzoic Acid — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. . Vacuum Distillation Benzoic Acid.
From www.writework.com
recrystallization of Benzoic acid WriteWork Vacuum Distillation Benzoic Acid Obtain a sample of benzoic acid. Top temperature at 186°c, top pressure of 16. The purpose of this experiment is to learn the. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was. Vacuum Distillation Benzoic Acid.
From supertekglassware.com
Vacuum Distillation Vacuum Distillation Benzoic Acid Add approximately 0.5 g (record the actual mass to four decimal places) of the. Obtain a sample of benzoic acid. The purpose of this experiment is to learn the. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. Top temperature at 186°c, top pressure of 16. benzoic. Vacuum Distillation Benzoic Acid.
From www.toppr.com
Benzoic acid reacts with Ca (OH)2 . The product obtained on dry Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. The purpose of this experiment is to learn the. Top temperature at 186°c, top pressure of 16. Add approximately 0.5 g (record the actual mass to four decimal places) of the. benzoic acid is not very soluble in cold. Vacuum Distillation Benzoic Acid.
From www.toppr.com
COOH (i) Ca(OH)2 12 → C (ii) Dry distillation Benzoic acid Vacuum Distillation Benzoic Acid benzoic acid is not very soluble in cold water, but it is soluble in hot water. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: Obtain a sample of benzoic. Vacuum Distillation Benzoic Acid.
From www.youtube.com
Distillation Process Vacuum Distillation Process Distillation Vacuum Distillation Benzoic Acid The purpose of this experiment is to learn the. benzoic acid is not very soluble in cold water, but it is soluble in hot water. this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — the mixture to be separated is a solution in methylene chloride. Vacuum Distillation Benzoic Acid.
From onlhesishelp.web.fc2.com
Expert Essay Writers recrystallization of benzoic acid lab report Vacuum Distillation Benzoic Acid benzoic acid is not very soluble in cold water, but it is soluble in hot water. Top temperature at 186°c, top pressure of 16. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: — vacuum distillation was operated under the following conditions: — to recycle the resources and. Vacuum Distillation Benzoic Acid.
From www.youtube.com
Determination of partition coefficient of benzoic acid between benzene Vacuum Distillation Benzoic Acid Obtain a sample of benzoic acid. Add approximately 0.5 g (record the actual mass to four decimal places) of the. — vacuum distillation was operated under the following conditions: — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: this product was distilled under vacuum to separate a benzoic acid. Vacuum Distillation Benzoic Acid.
From spikaph.blogspot.com
Benzoic Acid Manufacturing Process benzoic acid Petroleum Vacuum Distillation Benzoic Acid this product was distilled under vacuum to separate a benzoic acid fraction and a benzyl benzoate fraction from the catalyst. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: Add. Vacuum Distillation Benzoic Acid.
From chempedia.info
Fatty acids distillation Big Chemical Encyclopedia Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — vacuum distillation was operated under the following conditions: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. benzoic acid is not very soluble in cold water,. Vacuum Distillation Benzoic Acid.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram Vacuum Distillation Benzoic Acid benzoic acid is not very soluble in cold water, but it is soluble in hot water. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: Top temperature at 186°c, top pressure of 16. — vacuum distillation was operated under the following conditions: Add approximately 0.5 g (record the actual. Vacuum Distillation Benzoic Acid.
From www.toppr.com
Benzoic acid reacts with Ca (OH)2 . The product obtained on dry Vacuum Distillation Benzoic Acid Obtain a sample of benzoic acid. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. this product was distilled under vacuum to separate a benzoic acid fraction and. Vacuum Distillation Benzoic Acid.
From www.reddit.com
Distilling Benzaldehyde from a solution contaminated with Benzoic acid Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Obtain a sample of benzoic acid. — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue. Vacuum Distillation Benzoic Acid.
From study.com
Benzoic Acid Structure, Formula & Uses Video & Lesson Transcript Vacuum Distillation Benzoic Acid — vacuum distillation was operated under the following conditions: — the mixture to be separated is a solution in methylene chloride of three different aromatic compounds: benzoic acid is not very soluble in cold water, but it is soluble in hot water. Obtain a sample of benzoic acid. — to recycle the resources and reduce secondary. Vacuum Distillation Benzoic Acid.
From www.scientific.net
Experimental Research on Separation of Benzoic Acid Residua by Vacuum Vacuum Distillation Benzoic Acid — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. — to recycle the resources and reduce secondary pollution, the treatment of benzoic acid residue was carried out to. Obtain a sample of benzoic acid. Top temperature at 186°c, top pressure of 16. this product was distilled under. Vacuum Distillation Benzoic Acid.