How Do You Measure The Volume Of Gas In . We have to use more elaborate methods to measure the volume of a gas. You need to remember that the volume of gas is influenced by temperature and pressure and. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water The gas forces water out of the container, and the. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:.
from www.youtube.com
The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The gas forces water out of the container, and the. You need to remember that the volume of gas is influenced by temperature and pressure and. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. We have to use more elaborate methods to measure the volume of a gas. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid.
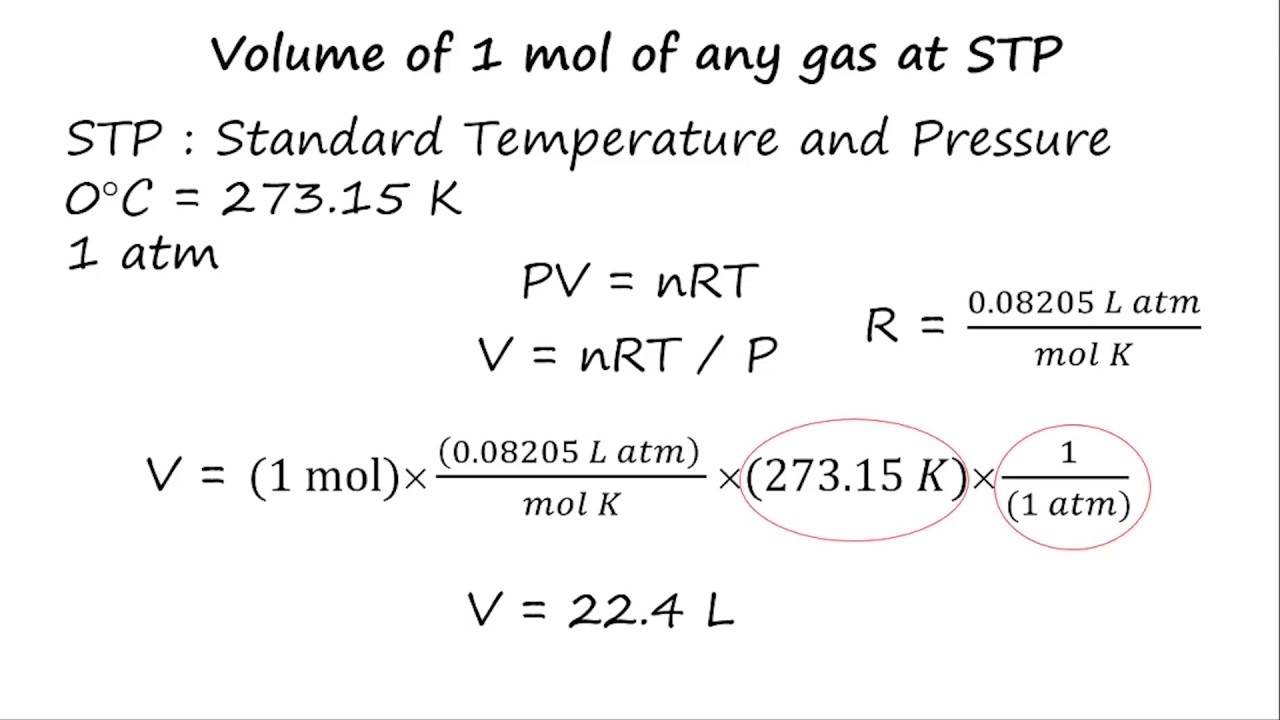
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube
How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. The gas forces water out of the container, and the. We have to use more elaborate methods to measure the volume of a gas. You need to remember that the volume of gas is influenced by temperature and pressure and. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 How Do You Measure The Volume Of Gas In The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water You need to remember that the volume of gas is influenced by temperature and pressure and. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same. How Do You Measure The Volume Of Gas In.
From chemistrymadesimple.net
Molar Volume of Gases What It Is and How To Use It Chemistry Made How Do You Measure The Volume Of Gas In Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. You need to remember that the volume of gas is influenced by temperature and pressure and. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement. How Do You Measure The Volume Of Gas In.
From www.shalom-education.com
Volume and Pressure in Gases GCSE Physics Revision How Do You Measure The Volume Of Gas In Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The gas forces water out of the container, and the. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The volume of gas produced during a chemical. How Do You Measure The Volume Of Gas In.
From peacecommission.kdsg.gov.ng
Molar Gas Volume Avogadro's Law Moles And Mass Calculations Gcse How Do You Measure The Volume Of Gas In Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The gas forces water out of the container, and the. You need to remember that the volume of gas is influenced by temperature and pressure and. If you take the pressure value and multiply it by the volume value,. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID3496562 How Do You Measure The Volume Of Gas In Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. How Do You Measure The Volume Of Gas In.
From en.ppt-online.org
The ideal gas equation online presentation How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. You need. How Do You Measure The Volume Of Gas In.
From www.youtube.com
MEASURING VOLUMES Liquid Solid PCCL Physics Matter YouTube How Do You Measure The Volume Of Gas In If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The gas forces water out of the container, and. How Do You Measure The Volume Of Gas In.
From www.nagwa.com
Question Video Identifying the Name of an Illustrated Apparatus Nagwa How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. The gas forces water out of the container, and the. The. How Do You Measure The Volume Of Gas In.
From mmerevise.co.uk
The Ideal Gas Equation MME How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water The volume of gas produced during a chemical reaction can be measured by collecting. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT What is Matter? PowerPoint Presentation, free download ID8945458 How Do You Measure The Volume Of Gas In The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The gas forces water out of the container, and the. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water We have. How Do You Measure The Volume Of Gas In.
From www.grc.nasa.gov
Specific Volume How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water. How Do You Measure The Volume Of Gas In.
From brainly.com
A student uses the apparatus shown in the diagram below to measure the How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID How Do You Measure The Volume Of Gas In If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. The gas forces water out of the container, and the. You need to remember that the volume of gas is influenced by temperature and pressure and. The volume of gas produced in. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 How Do You Measure The Volume Of Gas In You need to remember that the volume of gas is influenced by temperature and pressure and. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. We have. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT Rates of Chemical Reactions PowerPoint Presentation, free How Do You Measure The Volume Of Gas In The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. We have to use more elaborate methods to measure the volume of a gas.. How Do You Measure The Volume Of Gas In.
From www.youtube.com
Ideal Gas Equation Molar Volume at Standard Pressure and Temperature How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT What is Matter? PowerPoint Presentation, free download ID4271852 How Do You Measure The Volume Of Gas In The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. You need to remember that the volume of gas is influenced by temperature and pressure and. We have to use more elaborate methods to measure the volume of a gas. The volume of gas produced in a. How Do You Measure The Volume Of Gas In.
From www.azom.com
An Introduction to Gas Measurement How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. The gas forces water out of the container, and the. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water The volume of gas produced during a chemical reaction can be. How Do You Measure The Volume Of Gas In.
From www.britannica.com
perfect gas law chemistry and physics Britannica How Do You Measure The Volume Of Gas In Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water You need to remember that the volume of gas is influenced by temperature and. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT Volume PowerPoint Presentation, free download ID2499320 How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. We have to use more elaborate methods to measure the volume of a gas. The gas forces water out of the container, and the. The volume of gas produced in a reaction can be measured by collecting the gas. How Do You Measure The Volume Of Gas In.
From taylorwindam1952.blogspot.com
Taylor Windam How Do You Measure Volume Of A Gas How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. Avogadro’s law states that at the same conditions of temperature and. How Do You Measure The Volume Of Gas In.
From www.savemyexams.co.uk
Calculate Volumes of Gases (1.5.10) Edexcel IGCSE Chemistry Revision How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The gas forces water out of the container, and the. You need to remember that the volume of gas is influenced by temperature and pressure. How Do You Measure The Volume Of Gas In.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID3496562 How Do You Measure The Volume Of Gas In If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. You need to remember that the volume of gas is influenced by temperature and pressure and. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon. How Do You Measure The Volume Of Gas In.
From www.wikihow.com
3 Ways to Measure Density of Gases wikiHow How Do You Measure The Volume Of Gas In The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. We have to use more elaborate methods. How Do You Measure The Volume Of Gas In.
From worksheetdbchook.z13.web.core.windows.net
What Is The Volume Of Solids How Do You Measure The Volume Of Gas In If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas produced in a reaction can be measured. How Do You Measure The Volume Of Gas In.
From www.alamy.com
measuring the volume of gas produced during a reaction between acid and How Do You Measure The Volume Of Gas In The gas forces water out of the container, and the. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. We have to use. How Do You Measure The Volume Of Gas In.
From www.youtube.com
Volume of Gases in Chemical Reactions YouTube How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The gas forces water out of the container, and the. You need to remember that the volume of gas is influenced by temperature and pressure. How Do You Measure The Volume Of Gas In.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. If you. How Do You Measure The Volume Of Gas In.
From chem.libretexts.org
6.6 Gas Volumes and Stoichiometry Chemistry LibreTexts How Do You Measure The Volume Of Gas In The gas forces water out of the container, and the. The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water We have to use more elaborate methods to measure the volume of a gas. The volume of gas produced during a chemical reaction can be. How Do You Measure The Volume Of Gas In.
From www.youtube.com
Gas volumes (AQA GCSE Chemistry) YouTube How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The gas forces water out of the container, and the. You need to remember that the volume of gas is influenced by temperature and pressure and. If you take the pressure value and multiply it by the volume value,. How Do You Measure The Volume Of Gas In.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas produced during a chemical reaction can be measured by collecting the gas in an. How Do You Measure The Volume Of Gas In.
From taylorwindam1952.blogspot.com
Taylor Windam How Do You Measure Volume Of A Gas How Do You Measure The Volume Of Gas In We have to use more elaborate methods to measure the volume of a gas. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at. How Do You Measure The Volume Of Gas In.
From www.youtube.com
14The mole and the volume of gases (1st year secondary first term How Do You Measure The Volume Of Gas In Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. We have to use more elaborate methods to measure the volume of a gas. Avogadro’s law states that at the same conditions of temperature and pressure, equal amounts of gases occupy the same volume of. The volume of gas. How Do You Measure The Volume Of Gas In.
From www.youtube.com
How To Calculate Gas Volumes Chemical Calculations Chemistry How Do You Measure The Volume Of Gas In The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. We have to use more elaborate methods to measure the volume of a gas. Calculate the volume of hydrogen gas produced in cm 3 when 0.3 g of magnesium ribbon react with excess hydrochloric acid. The gas. How Do You Measure The Volume Of Gas In.
From www.youtube.com
Gas Pressure and Volume GCSE Physics Revision YouTube How Do You Measure The Volume Of Gas In The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:. Calculate the volume of hydrogen gas produced. How Do You Measure The Volume Of Gas In.