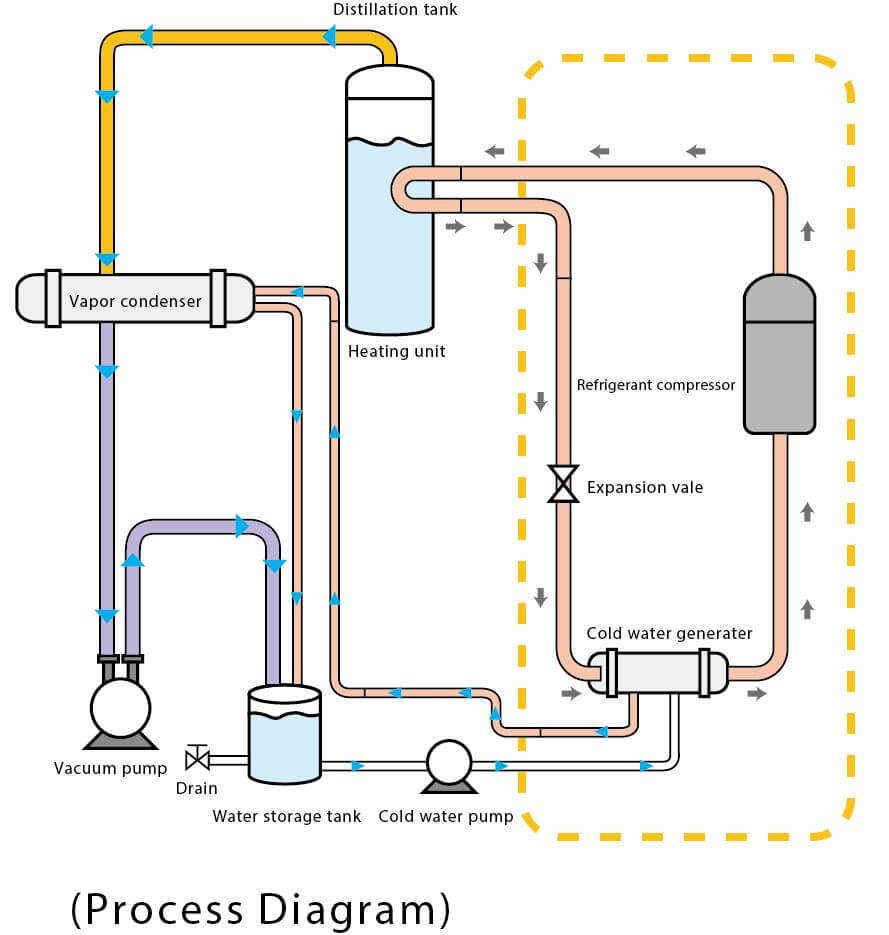
Vacuum Distillation Function . Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. In other words, distillation under reduced. When the pressure is lowered inside.
from www.fimitech.com.tw
In other words, distillation under reduced. When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant.
【Product】Vacuum distillation Taiwan Fimitech Corp.
Vacuum Distillation Function Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. When the pressure is lowered inside. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. In other words, distillation under reduced. Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be. Vacuum Distillation Function.
From en.wikipedia.org
Distillation Wikipedia Vacuum Distillation Function Vacuum distillation is a technique for the purification or separation of liquids or solvents. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). In other words, distillation under reduced. When. Vacuum Distillation Function.
From www.vecteezy.com
Distillation, distillation under reduced pressure, vacuum distillation Vacuum Distillation Function Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound. Vacuum Distillation Function.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). In other words, distillation under reduced. Vacuum. Vacuum Distillation Function.
From www.researchgate.net
Flow diagram of a dry vacuum distillation unit [9] Download Vacuum Distillation Function Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. When the pressure is lowered inside. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order. Vacuum Distillation Function.
From www.alamy.com
Vacuum Distillation System Stock Vector Image & Art Alamy Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less. Vacuum Distillation Function.
From oldmymages.blogspot.com
Vacuum Distillation Column P&id Oldmymages Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Vacuum distillation is a technique for the purification or separation of. Vacuum Distillation Function.
From www.researchgate.net
The schematic for the vacuum distillation process. Download Vacuum Distillation Function A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when. Vacuum Distillation Function.
From www.mechanical-knowledge.com
DISTILLATION COLUMN HOW IT WORK AND IT'S COMPONENTS Vacuum Distillation Function Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o. Vacuum Distillation Function.
From thepetrosolutions.com
Vacuum Distillation Unit in Oil Refinery The Petro Solutions Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. In other words, distillation under reduced. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents. When the pressure is lowered inside. Vacuum distillation is a. Vacuum Distillation Function.
From www.slideserve.com
PPT Chapter 4 Crude distillation PowerPoint Presentation, free Vacuum Distillation Function Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum. Vacuum Distillation Function.
From bbgate.com
Distillation and distillation systems Breaking Bad Vacuum Distillation Function A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation apparatus. Vacuum Distillation Function.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective Vacuum Distillation Function A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In other words, distillation under reduced. When the pressure. Vacuum Distillation Function.
From www.researchgate.net
Vacuum distillation apparatus. Download Scientific Diagram Vacuum Distillation Function A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation. Vacuum Distillation Function.
From www.fimitech.com.tw
【Product】Vacuum distillation Taiwan Fimitech Corp. Vacuum Distillation Function Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). In other words, distillation under reduced. Vacuum distillation is. Vacuum Distillation Function.
From www.britannica.com
distillation summary Britannica Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. When the pressure is lowered inside. A vacuum distillation is used when the boiling point of the compound (or the. Vacuum Distillation Function.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. Vacuum Distillation Function Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used. Vacuum Distillation Function.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Vacuum Distillation Function A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. When the pressure is lowered inside. Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation apparatus is shown in. Vacuum Distillation Function.
From www.alfalaval.sg
Vacuum distillation Alfa Laval Vacuum Distillation Function A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus. Vacuum Distillation Function.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific Vacuum Distillation Function In other words, distillation under reduced. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is performed by applying. Vacuum Distillation Function.
From www.mdpi.com
Entropy Free FullText Modeling and Simulation of an Energy Vacuum Distillation Function Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. In other words, distillation under reduced. When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure. Vacuum Distillation Function.
From www.indiamart.com
Vacuum Distillation Units at Rs 6000/piece Fractional Distillation Vacuum Distillation Function Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. In other words, distillation under reduced. A. Vacuum Distillation Function.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Vacuum Distillation Function In other words, distillation under reduced. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation. Vacuum Distillation Function.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Vacuum Distillation Function In other words, distillation under reduced. When the pressure is lowered inside. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum. Vacuum Distillation Function.
From www.researchgate.net
Fig. Atmosphere and Vacuum Distillation Download Scientific Diagram Vacuum Distillation Function When the pressure is lowered inside. In other words, distillation under reduced. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A vacuum distillation is performed by applying a vacuum source. Vacuum Distillation Function.
From www.dreamstime.com
Simple Distillation Apparatus Diagram with Full Process Vector Vacuum Distillation Function A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In other words, distillation under reduced. A vacuum distillation is. Vacuum Distillation Function.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Vacuum Distillation Function When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum. Vacuum Distillation Function.
From www.numerade.com
SOLVED The apparatus for vacuum distillation is essentially the same Vacuum Distillation Function Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A distillation operation that is. Vacuum Distillation Function.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Vacuum Distillation Function Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum. Vacuum Distillation Function.
From www.youtube.com
Vacuum Distillation YouTube Vacuum Distillation Function A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. In other words, distillation under reduced. When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a method of distillation whereby the. Vacuum Distillation Function.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Vacuum Distillation Function Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). When the pressure is lowered inside. In other words, distillation under reduced. Vacuum distillation is a method of distillation whereby. Vacuum Distillation Function.
From jupiter.plymouth.edu
DISTILLATION APPARATUS Vacuum Distillation Function A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents. When the pressure is lowered inside. A vacuum distillation is used when the boiling point. Vacuum Distillation Function.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' Vacuum Distillation Function In other words, distillation under reduced. When the pressure is lowered inside. Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a method of distillation whereby the pressure above. Vacuum Distillation Function.
From www.alibaba.com
(keda) Lab Vacuum Distillation Unit For Acetone Buy Vacuum Vacuum Distillation Function Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum. Vacuum Distillation Function.
From distillation.en.made-in-china.com
Lab MultiFunction Distillation Alcohol Rotatory Evaporator with Vacuum Distillation Function A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Boiling commences when the vapor pressure of a liquid or solution equals the. Vacuum Distillation Function.