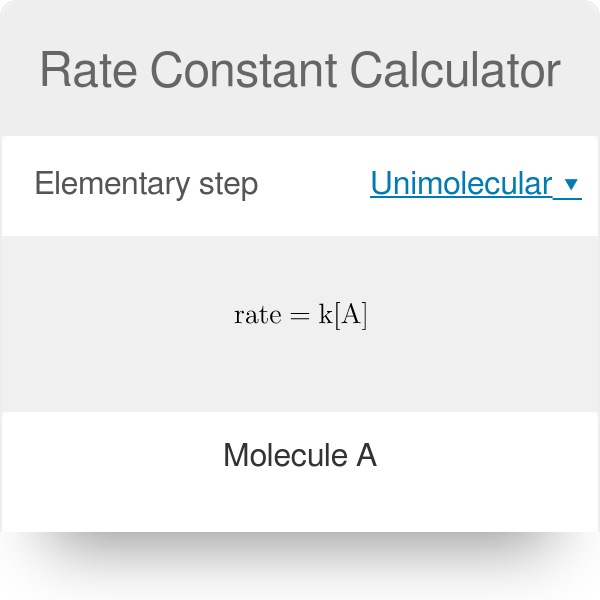
Rate Constant Calculation Examples . Use rate laws to calculate reaction rates. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: Rate = k [a]x [b]y [c]z. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Explain the form and function of a rate law. If the rate constant doubles, for example,. The rate of the reaction is given by the equation below, where. How to calculate rate constant. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Use rate and concentration data to identify reaction orders. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law.
from mybios.me
Use rate laws to calculate reaction rates. If the rate constant doubles, for example,. How to calculate rate constant. The rate of the reaction is given by the equation below, where. The rateconstant (k) of a reaction can be calculated using: The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Rate = k [a]x [b]y [c]z. Use rate and concentration data to identify reaction orders.
Convert Equation To Table Of Values Calculator Bios Pics
Rate Constant Calculation Examples The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: Use rate and concentration data to identify reaction orders. The rate of the reaction is given by the equation below, where. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Rate = k [a]x [b]y [c]z. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. How to calculate rate constant. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant Calculation Examples Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: How to calculate rate constant. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. The. Rate Constant Calculation Examples.
From www.researchgate.net
The calculated rate constants as a function of pressures. The Rate Constant Calculation Examples How to calculate rate constant. Use rate and concentration data to identify reaction orders. If the rate constant doubles, for example,. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Explain the form and function of a rate law. The rate of the reaction is given by. Rate Constant Calculation Examples.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant Calculation Examples The rateconstant (k) of a reaction can be calculated using: How to calculate rate constant. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. The initialrates and the rate equation. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Use. Rate Constant Calculation Examples.
From www.slideserve.com
PPT Ch. 12 Rates and Rate Laws PowerPoint Presentation, free download Rate Constant Calculation Examples Rate = k [a]x [b]y [c]z. The initialrates and the rate equation. How to calculate rate constant. If the rate constant doubles, for example,. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Use rate and concentration data to identify reaction orders. Explain the form and function. Rate Constant Calculation Examples.
From www.youtube.com
Rate constant calculation YouTube Rate Constant Calculation Examples Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical. Rate Constant Calculation Examples.
From www.coursehero.com
[Solved] Using the data in the table, calculate the rate constant of Rate Constant Calculation Examples The rate of the reaction is given by the equation below, where. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Use rate laws to. Rate Constant Calculation Examples.
From askfilo.com
The unit of rate constant for the reaction 2H2 +2NO→2H2 O+N2 which has r.. Rate Constant Calculation Examples Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. The rateconstant (k) of a reaction can be calculated using: The rate of the reaction is given by the equation below, where. How to calculate rate constant. Prepare a 0.100m solution butyl chloride in water and then measure. Rate Constant Calculation Examples.
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate Rate Constant Calculation Examples Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in. Rate Constant Calculation Examples.
From www.chegg.com
Solved Calculate the rate constant with proper units. Rate Constant Calculation Examples The rateconstant (k) of a reaction can be calculated using: The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Rate constant is the proportionality constant in the equation that expresses the relationship between the. Rate Constant Calculation Examples.
From mybios.me
Convert Equation To Table Of Values Calculator Bios Pics Rate Constant Calculation Examples The rate of the reaction is given by the equation below, where. Rate = k [a]x [b]y [c]z. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. Use rate laws to calculate reaction rates. How to calculate rate constant. The relation between the rate of a reaction and the concentrations of reactants. Rate Constant Calculation Examples.
From slideplayer.com
Reaction Rates and Equilibrium ppt download Rate Constant Calculation Examples The initialrates and the rate equation. Rate = k [a]x [b]y [c]z. If the rate constant doubles, for example,. Use rate laws to calculate reaction rates. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Rate constant is the proportionality constant in the equation that expresses the. Rate Constant Calculation Examples.
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 Rate Constant Calculation Examples Use rate and concentration data to identify reaction orders. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. How to calculate rate constant. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Explain the form and function. Rate Constant Calculation Examples.
From education-portal.com
Rate Constant and Rate Laws Video & Lesson Transcript Rate Constant Calculation Examples Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. Rate = k [a]x [b]y [c]z. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. If the rate constant doubles, for example,. Rate constant is the proportionality constant in. Rate Constant Calculation Examples.
From www.chegg.com
Solved Calculate the rate constant for Set A using the rates Rate Constant Calculation Examples Explain the form and function of a rate law. Rate = k [a]x [b]y [c]z. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. The initialrates and the rate equation. How to calculate rate. Rate Constant Calculation Examples.
From facts.net
14 Fascinating Facts About Rate Constant Rate Constant Calculation Examples The rate of the reaction is given by the equation below, where. Rate = k [a]x [b]y [c]z. How to calculate rate constant. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Explain the form and function of a rate law. The rateconstant (k) of a reaction. Rate Constant Calculation Examples.
From www.slideserve.com
PPT Calculations PowerPoint Presentation ID329325 Rate Constant Calculation Examples If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Rate = k [a]x [b]y [c]z. The rate of the reaction is given by the equation below, where. The initialrates and the rate equation. Use rate and. Rate Constant Calculation Examples.
From www.pinterest.com
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Rate Constant Calculation Examples Rate = k [a]x [b]y [c]z. Use rate and concentration data to identify reaction orders. Use rate laws to calculate reaction rates. The rateconstant (k) of a reaction can be calculated using: Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. How to calculate rate constant. Explain. Rate Constant Calculation Examples.
From brainly.com
Which equation would be used to calculate the rate constant from Rate Constant Calculation Examples How to calculate rate constant. Rate = k [a]x [b]y [c]z. Explain the form and function of a rate law. The rateconstant (k) of a reaction can be calculated using: The rate of the reaction is given by the equation below, where. The initialrates and the rate equation. Prepare a 0.100m solution butyl chloride in water and then measure the. Rate Constant Calculation Examples.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant Calculation Examples Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. The initialrates and the rate equation. Rate = k [a]x [b]y [c]z. The rate of the reaction is given. Rate Constant Calculation Examples.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant Calculation Examples Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: Use rate and concentration data to identify reaction orders. Prepare a 0.100m solution butyl chloride in water and then measure the concentration. Rate Constant Calculation Examples.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant Calculation Examples Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. If the rate constant doubles, for example,. Use rate laws to calculate reaction rates. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. The rate of the reaction. Rate Constant Calculation Examples.
From www.showme.com
ShowMe calculate the rate constant Rate Constant Calculation Examples The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Use rate and concentration data to identify reaction orders. Rate = k [a]x [b]y [c]z. If the rate constant doubles, for example,. How to calculate rate constant. Explain the form and function of a rate law. The initialrates and the rate. Rate Constant Calculation Examples.
From www.youtube.com
First Order Elimination Rate Constant and Halflife A closer look Rate Constant Calculation Examples If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: Rate = k [a]x [b]y [c]z. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Use rate and concentration data to identify reaction orders. Rate constant is the proportionality constant. Rate Constant Calculation Examples.
From www.bol.com
Rate Constant Calculation For Thermal Reactions 9780470582305 H Rate Constant Calculation Examples If the rate constant doubles, for example,. The initialrates and the rate equation. Use rate and concentration data to identify reaction orders. The rateconstant (k) of a reaction can be calculated using: Use rate laws to calculate reaction rates. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in. Rate Constant Calculation Examples.
From classnotes.org.in
Integrated Rate Expression Chemical Chemistry, Class 12 Rate Constant Calculation Examples Rate = k [a]x [b]y [c]z. Explain the form and function of a rate law. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The initialrates and the rate equation. Use rate and concentration data to identify reaction orders. How to calculate rate constant. Prepare a 0.100m solution butyl chloride in water. Rate Constant Calculation Examples.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant Calculation Examples If the rate constant doubles, for example,. The initialrates and the rate equation. Use rate and concentration data to identify reaction orders. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Use rate laws to calculate reaction rates. Explain the form and function of a rate law.. Rate Constant Calculation Examples.
From slideplayer.com
Reaction Rates and Equilibrium ppt download Rate Constant Calculation Examples Use rate and concentration data to identify reaction orders. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. The rateconstant (k) of a reaction can be calculated using: Explain the form and function of a rate law. Rate = k [a]x [b]y [c]z. The rate of the. Rate Constant Calculation Examples.
From slideplayer.com
AP Chemistry Chapter 14 Jeopardy ppt download Rate Constant Calculation Examples Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. Use rate and concentration data to identify reaction orders. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. How to calculate rate constant. The rate of the reaction is given by. Rate Constant Calculation Examples.
From www.youtube.com
Reaction Rates and Stoichiometry Chemistry Tutorial YouTube Rate Constant Calculation Examples The rate of the reaction is given by the equation below, where. The initialrates and the rate equation. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Rate constant is the proportionality constant in. Rate Constant Calculation Examples.
From chart-studio.plotly.com
Calculation of PseudoFirstOrder Rate Constant scatter chart made by Rate Constant Calculation Examples If the rate constant doubles, for example,. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. The initialrates and the rate equation. The rate of the reaction is given by the equation below, where. The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law.. Rate Constant Calculation Examples.
From www.youtube.com
first order reaction rate calculation example YouTube Rate Constant Calculation Examples The rate of the reaction is given by the equation below, where. If the rate constant doubles, for example,. Use rate laws to calculate reaction rates. The initialrates and the rate equation. How to calculate rate constant. The rateconstant (k) of a reaction can be calculated using: Rate = k [a]x [b]y [c]z. Rate constant is the proportionality constant in. Rate Constant Calculation Examples.
From www.numerade.com
SOLVED THE RATE CONSTANT UNITS You can now use the rate equation to Rate Constant Calculation Examples The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Explain the form and function of a rate law. The rate of the reaction is given by the equation below, where. The rateconstant (k) of a reaction can be calculated using: Rate = k [a]x [b]y [c]z. Use rate and concentration. Rate Constant Calculation Examples.
From www.youtube.com
Rate equation and the units of the rate constant YouTube Rate Constant Calculation Examples If the rate constant doubles, for example,. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Rate = k [a]x [b]y [c]z. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the. The initialrates and the rate equation. The relation between. Rate Constant Calculation Examples.
From www.meritnation.com
Rate constant of a first order reaction is 2 3*10^4 s^ calculate the Rate Constant Calculation Examples Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the.. Rate Constant Calculation Examples.
From www.coursehero.com
[Solved] . Example Seeded BOD Calculation. Calculate the B.O.D. of a Rate Constant Calculation Examples Prepare a 0.100m solution butyl chloride in water and then measure the concentration at various intervals when it is involved in the. If the rate constant doubles, for example,. Use rate and concentration data to identify reaction orders. The rateconstant (k) of a reaction can be calculated using: Rate constant is the proportionality constant in the equation that expresses the. Rate Constant Calculation Examples.