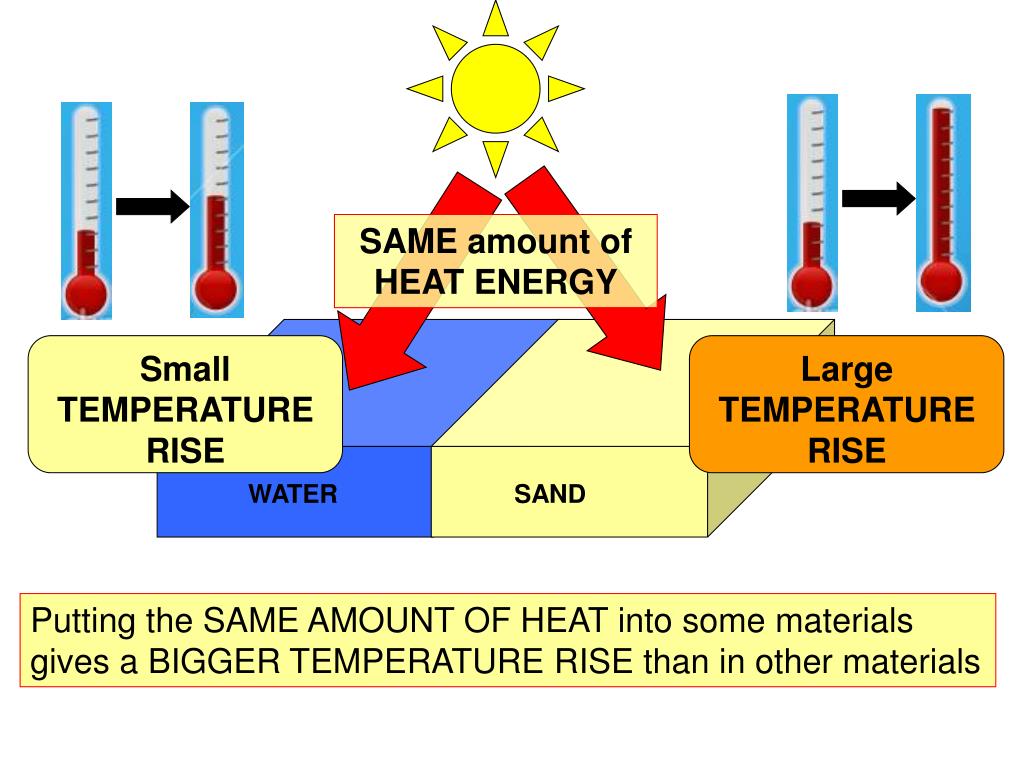
What Is Heat Capacity Effect . Heat capacity, ratio of heat absorbed by a material to the temperature change. The specific heat of a substance can also be described in terms of its molar amount. These occur at temperatures where the substance undergoes phase. Q = mcδt, where q is the symbol. In that case, we use a. The quantitative relationship between heat transfer and temperature change contains all three factors: The heat cap acity (\(c\)) of a body of. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Heat transfer and temperature change. We now introduce two concepts useful in describing heat flow and temperature change. It is usually expressed as calories per degree in.
from www.slideserve.com
The heat cap acity (\(c\)) of a body of. Heat capacity, ratio of heat absorbed by a material to the temperature change. These occur at temperatures where the substance undergoes phase. We now introduce two concepts useful in describing heat flow and temperature change. Heat transfer and temperature change. In that case, we use a. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. It is usually expressed as calories per degree in. The specific heat of a substance can also be described in terms of its molar amount. The quantitative relationship between heat transfer and temperature change contains all three factors:
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download
What Is Heat Capacity Effect Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Q = mcδt, where q is the symbol. These occur at temperatures where the substance undergoes phase. We now introduce two concepts useful in describing heat flow and temperature change. The heat cap acity (\(c\)) of a body of. It is usually expressed as calories per degree in. Heat transfer and temperature change. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat of a substance can also be described in terms of its molar amount. In that case, we use a. Heat capacity, ratio of heat absorbed by a material to the temperature change.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download What Is Heat Capacity Effect It is usually expressed as calories per degree in. Q = mcδt, where q is the symbol. The heat cap acity (\(c\)) of a body of. Heat transfer and temperature change. In that case, we use a. The specific heat of a substance can also be described in terms of its molar amount. The quantitative relationship between heat transfer and. What Is Heat Capacity Effect.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Heat Capacity Effect In that case, we use a. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. Q = mcδt, where q is the symbol. It is usually expressed as calories per degree in. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Heat capacity, ratio of heat absorbed by. What Is Heat Capacity Effect.
From courses.lumenlearning.com
Specific Heat Boundless Physics What Is Heat Capacity Effect The specific heat of a substance can also be described in terms of its molar amount. It is usually expressed as calories per degree in. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat capacity is intensive, and does not. What Is Heat Capacity Effect.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Heat Capacity Effect In that case, we use a. The heat cap acity (\(c\)) of a body of. Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. We now introduce two concepts useful in describing heat flow and temperature change. The quantitative relationship between heat transfer and temperature change contains. What Is Heat Capacity Effect.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Heat Capacity Effect The specific heat of a substance can also be described in terms of its molar amount. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer and temperature change. Q = mcδt, where q is the symbol. These occur at temperatures where the substance undergoes phase. The unit of heat capacity is j/ ∘ c. What Is Heat Capacity Effect.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Is Heat Capacity Effect Q = mcδt, where q is the symbol. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. It is usually expressed as calories per degree in. Heat transfer and temperature change. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The quantitative. What Is Heat Capacity Effect.
From pt.slideshare.net
Heat Capacity What Is Heat Capacity Effect The heat cap acity (\(c\)) of a body of. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The quantitative relationship between heat transfer and temperature change contains all three factors: In that case, we use a. Heat transfer and temperature change. These occur at temperatures where the substance undergoes phase. We. What Is Heat Capacity Effect.
From www.tec-science.com
Specific heat capacity of selected substances tecscience What Is Heat Capacity Effect The heat cap acity (\(c\)) of a body of. The specific heat of a substance can also be described in terms of its molar amount. Heat transfer and temperature change. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. Heat capacity, ratio of heat absorbed by a material to the temperature change. Q = mcδt, where. What Is Heat Capacity Effect.
From www.youtube.com
Specific heat capacity of solid by the method of mixture YouTube What Is Heat Capacity Effect These occur at temperatures where the substance undergoes phase. Q = mcδt, where q is the symbol. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat cap acity (\(c\)) of a body of. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat capacity is intensive, and does. What Is Heat Capacity Effect.
From studymind.co.uk
Heat Capacity Study Mind What Is Heat Capacity Effect Heat capacity, ratio of heat absorbed by a material to the temperature change. We now introduce two concepts useful in describing heat flow and temperature change. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. The heat cap acity (\(c\)) of a body of. Q =. What Is Heat Capacity Effect.
From www.studypool.com
SOLUTION What is Heat Capacity with sample solved problems. PPT What Is Heat Capacity Effect Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. These occur at temperatures where the substance undergoes phase. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. The quantitative relationship between heat transfer. What Is Heat Capacity Effect.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Effect The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The heat cap acity (\(c\)) of a body of. Heat transfer and temperature change. It is usually expressed as calories per degree in. The quantitative relationship between heat transfer and temperature change contains all three factors: In that case, we use a. The. What Is Heat Capacity Effect.
From chemistry101efhs.weebly.com
Heat vs. Temperature Chemistry 101 What Is Heat Capacity Effect The specific heat of a substance can also be described in terms of its molar amount. Q = mcδt, where q is the symbol. In that case, we use a. The heat cap acity (\(c\)) of a body of. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two. What Is Heat Capacity Effect.
From www.slideserve.com
PPT Chapter 17 Thermal Properties PowerPoint Presentation, free What Is Heat Capacity Effect Q = mcδt, where q is the symbol. The specific heat of a substance can also be described in terms of its molar amount. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The heat cap acity (\(c\)) of a body of. The quantitative relationship between heat transfer and temperature change contains all three factors: We. What Is Heat Capacity Effect.
From studylib.net
1.3 Specific Heat Capacity What Is Heat Capacity Effect It is usually expressed as calories per degree in. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. These occur at temperatures where the substance undergoes phase. The specific heat of a substance can also be described in terms of its molar amount. In that case, we use a. The unit of. What Is Heat Capacity Effect.
From www.slideserve.com
PPT Chapter 17 Thermal Properties PowerPoint Presentation, free What Is Heat Capacity Effect Heat transfer and temperature change. In that case, we use a. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive,. What Is Heat Capacity Effect.
From www.slideserve.com
PPT Properties of Materials PowerPoint Presentation, free download What Is Heat Capacity Effect The specific heat of a substance can also be described in terms of its molar amount. Heat capacity, ratio of heat absorbed by a material to the temperature change. The quantitative relationship between heat transfer and temperature change contains all three factors: The unit of heat capacity is j/ ∘ c or cal/ ∘ c. It is usually expressed as. What Is Heat Capacity Effect.
From www.youtube.com
Specific heat capacity YouTube What Is Heat Capacity Effect The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the. What Is Heat Capacity Effect.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID What Is Heat Capacity Effect Q = mcδt, where q is the symbol. It is usually expressed as calories per degree in. The specific heat of a substance can also be described in terms of its molar amount. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. In that case, we. What Is Heat Capacity Effect.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is Heat Capacity Effect The heat cap acity (\(c\)) of a body of. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat of a substance can also be described in terms of its molar amount. We now introduce two concepts. What Is Heat Capacity Effect.
From www.studypool.com
SOLUTION Physics notes heat capacity and specific heat capacity What Is Heat Capacity Effect Heat capacity, ratio of heat absorbed by a material to the temperature change. The quantitative relationship between heat transfer and temperature change contains all three factors: It is usually expressed as calories per degree in. The heat cap acity (\(c\)) of a body of. The heat capacity is a smooth, continuous function of temperature except for a small number of. What Is Heat Capacity Effect.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Heat Capacity Effect The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer and temperature change. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. The heat capacity is a smooth,. What Is Heat Capacity Effect.
From animalia-life.club
Examples Of Heat Energy What Is Heat Capacity Effect Q = mcδt, where q is the symbol. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The quantitative relationship between heat transfer and temperature change contains all three factors: The heat cap acity (\(c\)) of a body of. The unit of heat capacity is j/ ∘ c or cal/ ∘ c.. What Is Heat Capacity Effect.
From serc.carleton.edu
Heat Capacity What Is Heat Capacity Effect These occur at temperatures where the substance undergoes phase. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat cap acity (\(c\)) of a body of. Q = mcδt, where q is the symbol. It is usually expressed as calories per degree in. The specific heat of a substance can also be described in terms. What Is Heat Capacity Effect.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint What Is Heat Capacity Effect The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The quantitative relationship between heat transfer and temperature change contains all three factors: We now introduce two concepts useful in describing heat flow and temperature change. It is usually expressed as calories per degree in. Heat transfer and temperature change. Q = mcδt, where q is the. What Is Heat Capacity Effect.
From en.ppt-online.org
Class water online presentation What Is Heat Capacity Effect These occur at temperatures where the substance undergoes phase. In that case, we use a. We now introduce two concepts useful in describing heat flow and temperature change. The specific heat of a substance can also be described in terms of its molar amount. Heat transfer and temperature change. The specific heat capacity is intensive, and does not depend on. What Is Heat Capacity Effect.
From www.slideshare.net
Heat Capacity What Is Heat Capacity Effect The quantitative relationship between heat transfer and temperature change contains all three factors: Heat capacity, ratio of heat absorbed by a material to the temperature change. We now introduce two concepts useful in describing heat flow and temperature change. These occur at temperatures where the substance undergoes phase. Heat transfer and temperature change. It is usually expressed as calories per. What Is Heat Capacity Effect.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free What Is Heat Capacity Effect The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. We now introduce two concepts useful in. What Is Heat Capacity Effect.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Heat Capacity Effect The heat cap acity (\(c\)) of a body of. It is usually expressed as calories per degree in. These occur at temperatures where the substance undergoes phase. We now introduce two concepts useful in describing heat flow and temperature change. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer and temperature change. Heat capacity,. What Is Heat Capacity Effect.
From mavink.com
Formula Of Specific Heat Capacity What Is Heat Capacity Effect The unit of heat capacity is j/ ∘ c or cal/ ∘ c. In that case, we use a. The heat cap acity (\(c\)) of a body of. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. It is usually expressed as calories per degree in. We now introduce two concepts useful. What Is Heat Capacity Effect.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Heat Capacity Effect The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. These occur at temperatures where the substance undergoes phase. Q = mcδt, where q is the symbol. The specific heat of a substance can also be described in terms of its molar amount. Heat transfer and temperature. What Is Heat Capacity Effect.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Heat Capacity Effect Heat transfer and temperature change. These occur at temperatures where the substance undergoes phase. Heat capacity, ratio of heat absorbed by a material to the temperature change. Q = mcδt, where q is the symbol. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. In that. What Is Heat Capacity Effect.
From www.youtube.com
Lecture 14 What is Heat Capacity? YouTube What Is Heat Capacity Effect We now introduce two concepts useful in describing heat flow and temperature change. In that case, we use a. The quantitative relationship between heat transfer and temperature change contains all three factors: The heat cap acity (\(c\)) of a body of. The specific heat of a substance can also be described in terms of its molar amount. Heat capacity, ratio. What Is Heat Capacity Effect.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Is Heat Capacity Effect The unit of heat capacity is j/ ∘ c or cal/ ∘ c. The specific heat of a substance can also be described in terms of its molar amount. The heat cap acity (\(c\)) of a body of. We now introduce two concepts useful in describing heat flow and temperature change. Heat capacity, ratio of heat absorbed by a material. What Is Heat Capacity Effect.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is Heat Capacity Effect Heat transfer and temperature change. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of. It is usually expressed as calories per degree in. Q = mcδt, where q is the symbol. The specific heat of a substance can also be described in terms of its molar. What Is Heat Capacity Effect.