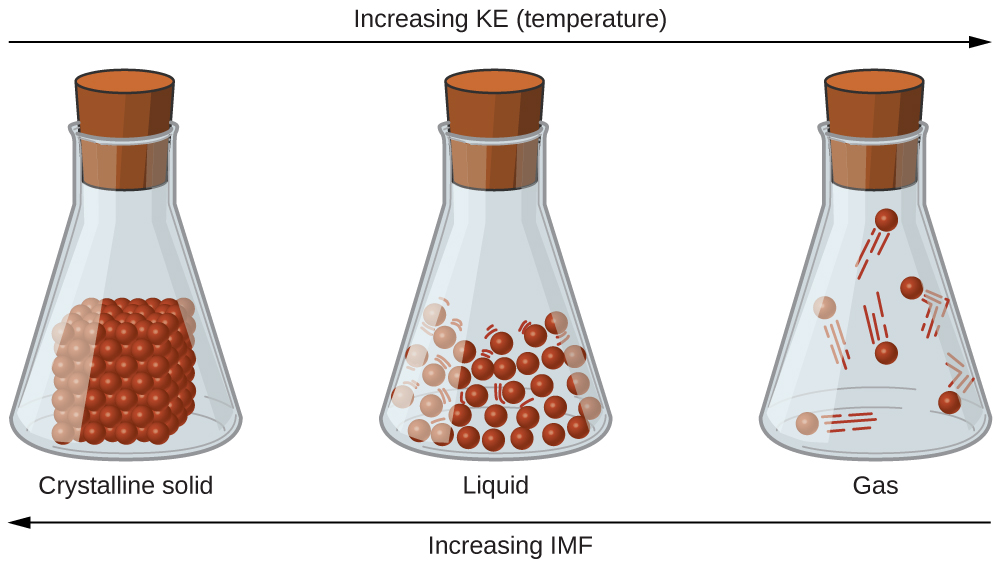
Why Are The Particles In A Liquid Held Together . The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The particles are held together too strongly to allow much movement but the particles do vibrate. Liquid are close together with no regular arrangement. The particles of a liquid remain. Gas are well separated with no regular arrangement. The liquid itself is held together by its own cohesive forces. In liquids, particles are quite close. The particles are held together too strongly to allow much movement but the particles do vibrate. In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and.
from philschatz.com
In liquids, particles are quite close. The particles of a liquid remain. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. Liquid are close together with no regular arrangement. When the weight of the liquid in the tube generates a downward force equal to. The liquid itself is held together by its own cohesive forces. Gas are well separated with no regular arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate.
Intermolecular Forces · Chemistry
Why Are The Particles In A Liquid Held Together Gas are well separated with no regular arrangement. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. In liquids, particles are quite close. Liquid are close together with no regular arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. When the weight of the liquid in the tube generates a downward force equal to. The particles are held together too strongly to allow much movement but the particles do vibrate. Gas are well separated with no regular arrangement. The liquid itself is held together by its own cohesive forces. The particles of a liquid remain. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. In liquids, particles are quite close.
From www.goodscience.com.au
Particles in Solids, Liquids and Gases Good Science Why Are The Particles In A Liquid Held Together The liquid itself is held together by its own cohesive forces. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. Liquid are close together with no. Why Are The Particles In A Liquid Held Together.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Why Are The Particles In A Liquid Held Together The particles are held together too strongly to allow much movement but the particles do vibrate. In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. When the weight of the liquid in the tube generates a downward force equal to. The particles. Why Are The Particles In A Liquid Held Together.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The Why Are The Particles In A Liquid Held Together Gas are well separated with no regular arrangement. In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. The liquid itself is held together by its own cohesive forces. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms). Why Are The Particles In A Liquid Held Together.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation ID542353 Why Are The Particles In A Liquid Held Together Liquid are close together with no regular arrangement. The particles of a liquid remain. In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. Gas are well. Why Are The Particles In A Liquid Held Together.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation Why Are The Particles In A Liquid Held Together When the weight of the liquid in the tube generates a downward force equal to. In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules. Why Are The Particles In A Liquid Held Together.
From primaryleap.co.uk
Chemistry Gases Level 1 activity for kids PrimaryLeap.co.uk Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. In liquids, particles are quite close. Liquid are close together with no regular arrangement. The liquid itself is held together by its own cohesive forces. The particles of a liquid remain. Gas are. Why Are The Particles In A Liquid Held Together.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. Liquid are close together with no regular arrangement. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The state of. Why Are The Particles In A Liquid Held Together.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Why Are The Particles In A Liquid Held Together The particles of a liquid remain. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. When the weight of the liquid in the tube generates a downward force equal to. Liquid are close together with no regular arrangement. The liquid itself is held together by its own cohesive. Why Are The Particles In A Liquid Held Together.
From www.teachoo.com
Characterstics of Particles of Matter Class 9 Science Notes Teacho Why Are The Particles In A Liquid Held Together Gas are well separated with no regular arrangement. In liquids, particles are quite close. Liquid are close together with no regular arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles of a liquid remain. The liquid itself is held together by its own cohesive forces. The state of a substance. Why Are The Particles In A Liquid Held Together.
From www.studocu.com
Particle Model of Matter It states that All matter (solids, liquids Why Are The Particles In A Liquid Held Together The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. Liquid are close together with no regular arrangement. The particles are held together too strongly to allow much. Why Are The Particles In A Liquid Held Together.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. The particles of a liquid remain. The particles are held together too strongly to allow much movement but the particles do vibrate. Liquid are close together with no regular arrangement. When the weight of the liquid in the tube generates a downward force equal to. The state of a substance depends on the balance. Why Are The Particles In A Liquid Held Together.
From www.nagwa.com
Question Video Explaining Why Particles Collide More Frequently When Why Are The Particles In A Liquid Held Together Gas are well separated with no regular arrangement. In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. The particles are held together too strongly to allow much movement but the particles do vibrate. In liquids, particles are quite close. The state of a substance depends on the balance. Why Are The Particles In A Liquid Held Together.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Why Are The Particles In A Liquid Held Together The liquid itself is held together by its own cohesive forces. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. Liquid are close together with no regular arrangement. Gas are well separated with no regular arrangement. In liquids, particles are quite close. The particles are held together. Why Are The Particles In A Liquid Held Together.
From socratic.org
How does the arrangement of the particles in a liquid compare to that Why Are The Particles In A Liquid Held Together The liquid itself is held together by its own cohesive forces. The particles are held together too strongly to allow much movement but the particles do vibrate. When the weight of the liquid in the tube generates a downward force equal to. In liquids, particles are quite close. The particles are held together too strongly to allow much movement but. Why Are The Particles In A Liquid Held Together.
From www.ep.ph.bham.ac.uk
Why study particle physics? A brief introduction Why Are The Particles In A Liquid Held Together The particles are held together too strongly to allow much movement but the particles do vibrate. When the weight of the liquid in the tube generates a downward force equal to. In liquids, particles are quite close. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. Gas are. Why Are The Particles In A Liquid Held Together.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Why Are The Particles In A Liquid Held Together Liquid are close together with no regular arrangement. When the weight of the liquid in the tube generates a downward force equal to. Gas are well separated with no regular arrangement. The particles of a liquid remain. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The liquid. Why Are The Particles In A Liquid Held Together.
From nftpanel.net
Which Particle Model Represents a Chemical Change Why Are The Particles In A Liquid Held Together The particles are held together too strongly to allow much movement but the particles do vibrate. Gas are well separated with no regular arrangement. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The state of a substance depends on the balance between the kinetic energy of the. Why Are The Particles In A Liquid Held Together.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Why Are The Particles In A Liquid Held Together Liquid are close together with no regular arrangement. When the weight of the liquid in the tube generates a downward force equal to. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The state of a substance depends on the balance between the kinetic energy of the individual. Why Are The Particles In A Liquid Held Together.
From royce-bogspotmccarty.blogspot.com
Describe the Movement of the Particles in the Air Why Are The Particles In A Liquid Held Together Liquid are close together with no regular arrangement. The liquid itself is held together by its own cohesive forces. Gas are well separated with no regular arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate. In liquids, particles are quite close. When the weight of the liquid in the tube generates a. Why Are The Particles In A Liquid Held Together.
From philschatz.com
Intermolecular Forces · Chemistry Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. Gas are well separated with no regular arrangement. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The liquid itself is held together by its own cohesive forces. The particles are held together too strongly to allow much movement but the particles. Why Are The Particles In A Liquid Held Together.
From byjus.com
What force holds atoms together? Why Are The Particles In A Liquid Held Together The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The liquid itself is held together by its own cohesive forces. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. In liquids, particles are quite close.. Why Are The Particles In A Liquid Held Together.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts Why Are The Particles In A Liquid Held Together The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles of a liquid remain. Gas are well separated with no regular arrangement. When the weight of the liquid in the tube. Why Are The Particles In A Liquid Held Together.
From socratic.org
What happens to the energy of its molecules as ice melts into Why Are The Particles In A Liquid Held Together The particles of a liquid remain. In liquids, particles are quite close. Gas are well separated with no regular arrangement. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The particles are held together too strongly to allow much movement but the particles do vibrate. The state of. Why Are The Particles In A Liquid Held Together.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The particles are held together too strongly to allow much movement but. Why Are The Particles In A Liquid Held Together.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Why Are The Particles In A Liquid Held Together Liquid are close together with no regular arrangement. In liquids, particles are quite close. The liquid itself is held together by its own cohesive forces. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The particles of a liquid remain. The state of a substance depends on the. Why Are The Particles In A Liquid Held Together.
From nenmongdangkim.com
In Which State Do Particles Have No Bonds Exploring The World Of Isolation Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The liquid itself is held together by its own cohesive forces. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles are held together too. Why Are The Particles In A Liquid Held Together.
From chemstuff.co.uk
Particle Model of Solids, Liquids and Gases Chemstuff Why Are The Particles In A Liquid Held Together The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. Gas are well separated with no regular arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles of a liquid remain. The particles of a solid are very close. Why Are The Particles In A Liquid Held Together.
From www.teachoo.com
Show Particles of Matter attract each other with an Activity Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The particles are held together too strongly to allow much movement but the particles do vibrate. The state of a substance depends on the balance between the kinetic energy of the individual. Why Are The Particles In A Liquid Held Together.
From courses.lumenlearning.com
The Dissolution Process Chemistry Why Are The Particles In A Liquid Held Together The particles of a liquid remain. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The liquid itself is held together by its own cohesive forces.. Why Are The Particles In A Liquid Held Together.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Why Are The Particles In A Liquid Held Together The liquid itself is held together by its own cohesive forces. In liquids, particles are quite close. The particles are held together too strongly to allow much movement but the particles do vibrate. Liquid are close together with no regular arrangement. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or. Why Are The Particles In A Liquid Held Together.
From www.teachoo.com
Arrange in increasing order of forces of attraction b/w particles Why Are The Particles In A Liquid Held Together The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. Gas are well separated with no regular arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles are held together too strongly to allow much movement but the particles do. Why Are The Particles In A Liquid Held Together.
From www.slideserve.com
PPT Particle Theory PowerPoint Presentation, free download Why Are The Particles In A Liquid Held Together Liquid are close together with no regular arrangement. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and the. In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. The particles of a liquid remain. The particles are. Why Are The Particles In A Liquid Held Together.
From www.len.com.ng
Liquid state of matter Characteristics of liquids Why Are The Particles In A Liquid Held Together When the weight of the liquid in the tube generates a downward force equal to. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The particles are. Why Are The Particles In A Liquid Held Together.
From www.goodscience.com.au
Particles in Solids, Liquids and Gases Good Science Why Are The Particles In A Liquid Held Together In liquids, particles are quite close. When the weight of the liquid in the tube generates a downward force equal to. The particles are held together too strongly to allow much movement but the particles do vibrate. The particles of a solid are very close together.it melts when it changes from the solid state to the liquid state. The liquid. Why Are The Particles In A Liquid Held Together.
From edurev.in
Gaseous Introduction (Part 1) Chemistry Notes EduRev Why Are The Particles In A Liquid Held Together The particles are held together too strongly to allow much movement but the particles do vibrate. In liquids, particles are quite close. In liquids, particles are quite close. The state of a substance depends on the balance between the kinetic energy of the individual particles (molecules or atoms) and. Gas are well separated with no regular arrangement. The liquid itself. Why Are The Particles In A Liquid Held Together.