Calorimeter Constant Is Determined By . Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. See examples, equations, and calculations for determining the calorimeter. Learn how to find the heat capacity of a calorimeter using different methods and examples. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Follow the steps to measure the temperature.
from www.youtube.com
Learn how to find the heat capacity of a calorimeter using different methods and examples. See examples, equations, and calculations for determining the calorimeter. Follow the steps to measure the temperature. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius.
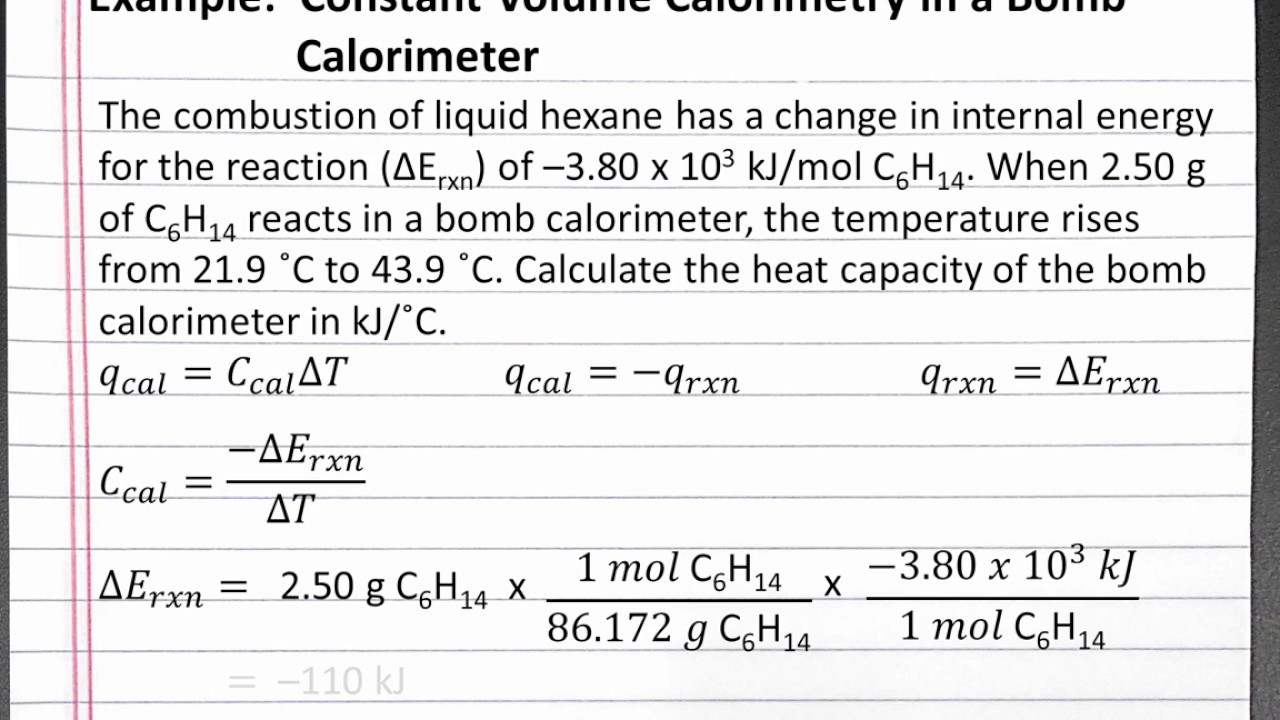
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube
Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Follow the steps to measure the temperature. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. See examples, equations, and calculations for determining the calorimeter. Learn how to find the heat capacity of a calorimeter using different methods and examples. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances.
From slideplayer.com
Enthalpy and Calorimetry ppt download Calorimeter Constant Is Determined By Follow the steps to measure the temperature. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Learn how to determine. Calorimeter Constant Is Determined By.
From www.scribd.com
calorimetry Calorimeter Constant Is Determined By The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. Follow the steps to measure the temperature. Learn how to determine it by mixing water. Calorimeter Constant Is Determined By.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Calorimeter Constant Is Determined By A calorimeter constant is the. See examples, equations, and calculations for determining the calorimeter. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. The mass of. Calorimeter Constant Is Determined By.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant Is Determined By Follow the steps to measure the temperature. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. This. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED When a solid dissolves in water; heat may be evolved or Calorimeter Constant Is Determined By This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. A calorimeter constant is the. Learn how to find the heat capacity of a calorimeter using different methods and examples.. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVEDUse the References to access important values if needed for this Calorimeter Constant Is Determined By Learn how to find the heat capacity of a calorimeter using different methods and examples. A calorimeter constant is the. Follow the steps to measure the temperature. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. See examples, equations, and calculations. Calorimeter Constant Is Determined By.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Follow the steps to measure the temperature. Learn how to find the heat capacity of a calorimeter using different methods and examples. The calorimeter constant is the amount of heat energy required to raise the temperature. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Constant Is Determined By This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. A calorimeter constant is the. Learn how to determine it by mixing water at different temperatures and how to use. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved determined is called the calorimeter constant. grams Calorimeter Constant Is Determined By See examples, equations, and calculations for determining the calorimeter. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Learn how to find the heat capacity of a calorimeter using different methods and examples. This web page explains the principles and applications of calorimetry, an experimental. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved Thermometer When a solid dissolves in water, heat may Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Follow the steps to measure the temperature. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. See examples, equations, and calculations for determining the. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED In the laboratory, a "coffee cup calorimeter" or constant Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. A calorimeter constant is the. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Follow the steps to measure the temperature. The mass of the bomb. Calorimeter Constant Is Determined By.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Calorimeter Constant Is Determined By The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. See examples, equations, and calculations for determining the calorimeter. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. A calorimeter constant is the. The. Calorimeter Constant Is Determined By.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Is Determined By The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. Follow the steps to measure the temperature. Learn how to find the heat capacity of a calorimeter using different methods and examples. See examples, equations, and calculations for determining the calorimeter. This. Calorimeter Constant Is Determined By.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calorimeter Constant Is Determined By The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. See examples, equations, and calculations for determining the calorimeter. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. A calorimeter. Calorimeter Constant Is Determined By.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Constant Is Determined By Learn how to find the heat capacity of a calorimeter using different methods and examples. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. See examples, equations, and calculations for determining the calorimeter. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED Abomb calorimeter; Or a constant volume calorimeter; is a Calorimeter Constant Is Determined By See examples, equations, and calculations for determining the calorimeter. A calorimeter constant is the. Learn how to find the heat capacity of a calorimeter using different methods and examples. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. The calorimeter constant. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved When a solid dissolves in water, heat may be evolved Calorimeter Constant Is Determined By The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. A calorimeter constant is the. See examples, equations, and calculations for determining the calorimeter. Follow the steps to measure the temperature. This web page explains the principles and applications of calorimetry, an. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved 2. A student determined the calorimeter constant of Calorimeter Constant Is Determined By This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. See examples, equations, and calculations for determining the calorimeter. Follow the steps to. Calorimeter Constant Is Determined By.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Is Determined By The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Learn how to find the heat capacity of a calorimeter using. Calorimeter Constant Is Determined By.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimeter Constant Is Determined By See examples, equations, and calculations for determining the calorimeter. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. Follow the steps to measure the. Calorimeter Constant Is Determined By.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant Is Determined By The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Learn how to find the heat capacity of a calorimeter using different methods and examples.. Calorimeter Constant Is Determined By.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter Constant Is Determined By A calorimeter constant is the. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Follow the steps to measure the temperature. See examples, equations, and calculations for determining the calorimeter. This web page explains the principles and applications of calorimetry, an experimental method to measure. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Calorimeter Constant Is Determined By The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Follow the steps to measure the temperature. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. Learn how to determine. Calorimeter Constant Is Determined By.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Constant Is Determined By A calorimeter constant is the. Follow the steps to measure the temperature. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to determine it by mixing water at different temperatures and how to. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Calorimeter Constant Is Determined By Learn how to find the heat capacity of a calorimeter using different methods and examples. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. A calorimeter constant is the. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the. Calorimeter Constant Is Determined By.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimeter Constant Is Determined By This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. See examples, equations, and calculations for determining the calorimeter. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to determine it by mixing water at different temperatures and how to use it to. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED In the laboratory a "coffee cup calorimeter, Or constant Calorimeter Constant Is Determined By The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. See examples, equations, and calculations for determining the calorimeter. The calorimeter constant is. Calorimeter Constant Is Determined By.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Constant Is Determined By See examples, equations, and calculations for determining the calorimeter. A calorimeter constant is the. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. Follow the steps. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, Or constant Calorimeter Constant Is Determined By Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances. See examples, equations, and calculations for determining the calorimeter. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The mass of the bomb multiplied by its. Calorimeter Constant Is Determined By.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Calorimeter Constant Is Determined By Follow the steps to measure the temperature. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to determine it by mixing water at different temperatures and how to use it to measure the. Calorimeter Constant Is Determined By.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Constant Is Determined By The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. A calorimeter constant is the. Follow the steps to measure the temperature. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree. Calorimeter Constant Is Determined By.
From saylordotorg.github.io
Calorimetry Calorimeter Constant Is Determined By A calorimeter constant is the. Follow the steps to measure the temperature. See examples, equations, and calculations for determining the calorimeter. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to determine it by mixing water at different temperatures and how to use it to measure the specific heat of other substances.. Calorimeter Constant Is Determined By.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Calorimeter Constant Is Determined By Learn how to find the heat capacity of a calorimeter using different methods and examples. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant, denoted by the symbol c with units of joules per degree celsius. Learn how to determine it by mixing water at different temperatures and how to use it to. Calorimeter Constant Is Determined By.