Copper Ii Chloride And Aluminum Equation . — in this video we will balance the equation al + cucl2 = alcl3 + cu. When a ball of aluminum foil is placed in a copper. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. — a ball of aluminum foil is added to an aqueous solution of copper (ii). the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules).
from www.chegg.com
Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — in this video we will balance the equation al + cucl2 = alcl3 + cu. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. When a ball of aluminum foil is placed in a copper. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). — a ball of aluminum foil is added to an aqueous solution of copper (ii).
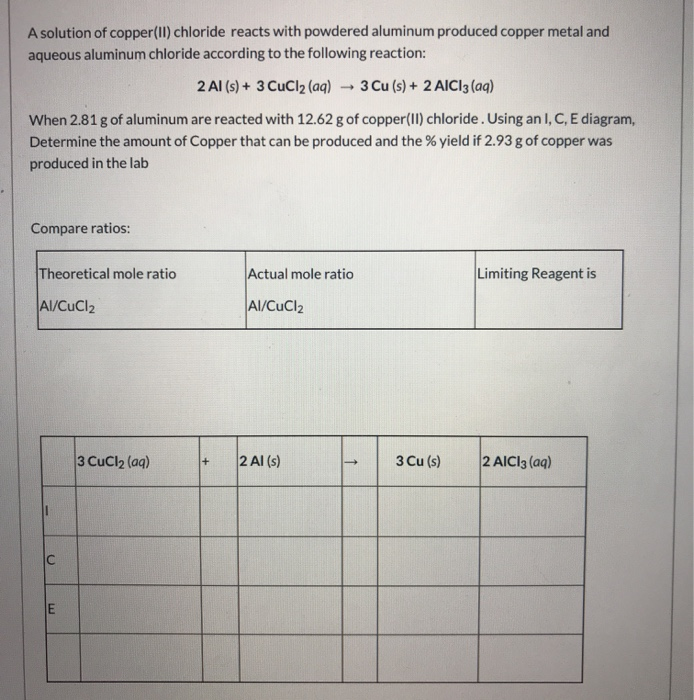
Solved A solution of copper(II) chloride reacts with
Copper Ii Chloride And Aluminum Equation Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. When a ball of aluminum foil is placed in a copper. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — in this video we will balance the equation al + cucl2 = alcl3 + cu. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). — a ball of aluminum foil is added to an aqueous solution of copper (ii). the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules).
From brainly.in
Aluminium plus copper chloride reacts to aluminium chloride plus copper Copper Ii Chloride And Aluminum Equation Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — in this video we will balance the equation al + cucl2 = alcl3 + cu. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. —. Copper Ii Chloride And Aluminum Equation.
From studylib.net
Reaction of Copper (II) Chloride and Aluminum Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. — a ball of aluminum foil is added to an aqueous solution of copper (ii). the chloride ion helps to separate the aluminum. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
Representing Reactions Aluminum and Copper (II) Chloride YouTube Copper Ii Chloride And Aluminum Equation — a ball of aluminum foil is added to an aqueous solution of copper (ii). using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
Aluminum and copper II chloride YouTube Copper Ii Chloride And Aluminum Equation — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — in this video we will. Copper Ii Chloride And Aluminum Equation.
From studylib.net
A Reaction between Copper II Chloride and Copper Ii Chloride And Aluminum Equation — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form. Copper Ii Chloride And Aluminum Equation.
From slideplayer.com
Unit 8 Chemical Reactions ppt download Copper Ii Chloride And Aluminum Equation — a ball of aluminum foil is added to an aqueous solution of copper (ii). using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). — in this video we will balance the equation al + cucl2 = alcl3 + cu. — therefore when aluminum. Copper Ii Chloride And Aluminum Equation.
From www.numerade.com
SOLVED a. Write a balanced molecular equation for the reaction of Copper Ii Chloride And Aluminum Equation Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — in this video we will balance the equation al + cucl2 = alcl3 + cu. — a ball of aluminum foil is added to an aqueous solution of copper (ii). the chloride ion helps to separate the aluminum. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
The Aluminum and Copper Chloride Reaction prelab YouTube Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. When a ball of aluminum foil is placed in a copper. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. — a ball of aluminum foil is added to an aqueous solution of. Copper Ii Chloride And Aluminum Equation.
From slideplayer.com
Chemical Reactions Intro to Reactions. ppt download Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — in this video we will balance the equation al + cucl2 = alcl3 + cu. — a ball of aluminum foil is added to an aqueous solution of copper (ii). using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict. Copper Ii Chloride And Aluminum Equation.
From www.chegg.com
Solved 5. Equimolar solutions of copper(II) chloride, Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). — a ball of aluminum foil is added to an aqueous solution of copper (ii). — therefore when aluminum foil is put into the. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
Aluminum and Copper (II) Chloride Reaction YouTube Copper Ii Chloride And Aluminum Equation Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — a ball of aluminum foil is added to an aqueous solution of copper (ii). When a ball of aluminum foil is placed in a copper. — in this video we will balance the equation al + cucl2 = alcl3. Copper Ii Chloride And Aluminum Equation.
From www.numerade.com
SOLVED Aluminum reacts with copper(II) chloride to produce copper and Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. When a ball of aluminum foil is placed in a copper. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — a ball of aluminum foil is added to an aqueous solution of copper (ii). — therefore. Copper Ii Chloride And Aluminum Equation.
From www.pw.live
Copper II Chloride Formula, Structure, Properties, Uses Copper Ii Chloride And Aluminum Equation the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — in this video we will balance the equation al + cucl2 = alcl3 +. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
Easy tips to balance Aluminium+ copper chloride= Aluminium chloride Copper Ii Chloride And Aluminum Equation — in this video we will balance the equation al + cucl2 = alcl3 + cu. the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal,. Copper Ii Chloride And Aluminum Equation.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Copper Ii Chloride And Aluminum Equation — a ball of aluminum foil is added to an aqueous solution of copper (ii). When a ball of aluminum foil is placed in a copper. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — therefore. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
Copper (ii) chloride Aluminium reaction YouTube Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. using the balanced chemical equation for. Copper Ii Chloride And Aluminum Equation.
From www.chegg.com
Solved A solution of copper(II) chloride reacts with Copper Ii Chloride And Aluminum Equation — a ball of aluminum foil is added to an aqueous solution of copper (ii). Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. — in this video we will balance the. Copper Ii Chloride And Aluminum Equation.
From www.chegg.com
Solved Aluminum metal reacts with aqueous copper (II) Copper Ii Chloride And Aluminum Equation — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — a ball of aluminum foil is added to an aqueous solution of copper (ii). using the balanced chemical equation for the reaction. Copper Ii Chloride And Aluminum Equation.
From studylib.net
Aluminum and Copper Chloride Activity Copper Ii Chloride And Aluminum Equation When a ball of aluminum foil is placed in a copper. the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). — a ball of aluminum foil is added to an aqueous solution of copper (ii). — therefore when aluminum foil is. Copper Ii Chloride And Aluminum Equation.
From dxouounyb.blob.core.windows.net
Mixing Aluminum With Copper(Ii) Chloride Temperature at Valerie Ohler blog Copper Ii Chloride And Aluminum Equation the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). — in this video we will balance the equation al + cucl2 = alcl3 + cu. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the. Copper Ii Chloride And Aluminum Equation.
From slideplayer.com
II. Balancing Equations ppt download Copper Ii Chloride And Aluminum Equation — a ball of aluminum foil is added to an aqueous solution of copper (ii). using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). When a ball of aluminum foil is placed in a copper. the chloride ion helps to separate the aluminum from the. Copper Ii Chloride And Aluminum Equation.
From slidetodoc.com
Balancing Chemical Equations What is a chemical equation Copper Ii Chloride And Aluminum Equation the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). — a ball of aluminum foil is added to an aqueous solution of copper (ii). Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — in this video we. Copper Ii Chloride And Aluminum Equation.
From studylib.net
Laboratory 5 Stoichiometry of Copper (II) Chloride and Aluminum Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — in this video we will balance the equation al + cucl2 = alcl3 + cu. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). — a ball of aluminum foil is added. Copper Ii Chloride And Aluminum Equation.
From www.numerade.com
SOLVED If we have 0.572 grams of aluminum and 3.029 grams of copper(II Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). When a ball. Copper Ii Chloride And Aluminum Equation.
From www.numerade.com
SOLVED Write balanced molecular equation for the redox reaction Copper Ii Chloride And Aluminum Equation Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. When a ball of aluminum foil is placed in a copper. — a ball of aluminum foil is added to an aqueous solution of copper (ii). — therefore when aluminum foil is put into the copper salt solution, aluminum atoms. Copper Ii Chloride And Aluminum Equation.
From ar.inspiredpencil.com
Copper Ii Chloride Lewis Structure Copper Ii Chloride And Aluminum Equation When a ball of aluminum foil is placed in a copper. — a ball of aluminum foil is added to an aqueous solution of copper (ii). — in this video we will balance the equation al + cucl2 = alcl3 + cu. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the. Copper Ii Chloride And Aluminum Equation.
From www.chegg.com
Solved 5. Equimolar solutions of copper(II) chloride, Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). the chloride ion helps to separate the. Copper Ii Chloride And Aluminum Equation.
From www.pinterest.com
A single replacement reaction involving aluminum and a solution of Copper Ii Chloride And Aluminum Equation — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). — a ball of aluminum foil is added to an. Copper Ii Chloride And Aluminum Equation.
From www.numerade.com
SOLVED If we have 0.572 grams of aluminum and 3.029 grams of copper(II Copper Ii Chloride And Aluminum Equation — therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. — a ball of aluminum foil is added to an aqueous solution of copper (ii). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
The electrolysis of copper (II) chloride. YouTube Copper Ii Chloride And Aluminum Equation — a ball of aluminum foil is added to an aqueous solution of copper (ii). the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). When a ball of aluminum foil is placed in a copper. Copper(ii) chloride dihydrate + aluminium = copper. Copper Ii Chloride And Aluminum Equation.
From www.youtube.com
Al + CuCl2= AlCl3 + Cu Balanced EquationAluminum+Copper chloride Copper Ii Chloride And Aluminum Equation Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — therefore when. Copper Ii Chloride And Aluminum Equation.
From www.chegg.com
Solved Copper (II) Chloride and Aluminum Reaction (done Copper Ii Chloride And Aluminum Equation When a ball of aluminum foil is placed in a copper. Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride. Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — in this video we will balance the equation al + cucl2 = alcl3 + cu. —. Copper Ii Chloride And Aluminum Equation.
From mackenzie-chapter.blogspot.com
Aluminum Copper Ii Chloride Reaction Lab Answers 50+ Pages Summary Doc Copper Ii Chloride And Aluminum Equation the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). Copper(ii) chloride dihydrate + aluminium = copper + water + aluminum chloride.. Copper Ii Chloride And Aluminum Equation.
From brainly.in
Write balanced chemical equations for the aluminium+copper chloride Copper Ii Chloride And Aluminum Equation the chloride ion helps to separate the aluminum from the oxygen so that the aluminum can react with the copper ions (and the water molecules). When a ball of aluminum foil is placed in a copper. — in this video we will balance the equation al + cucl2 = alcl3 + cu. — therefore when aluminum foil. Copper Ii Chloride And Aluminum Equation.
From ar.inspiredpencil.com
Copper Ii Chloride And Aluminum Copper Ii Chloride And Aluminum Equation using the balanced chemical equation for the reaction of aluminum and copper(ii) chloride, predict the number of (a) moles and (b). — a ball of aluminum foil is added to an aqueous solution of copper (ii). Aluminum metal reacts with aqueous copper(ii) chloride dihydrate (cucl 2 ·2h 2 o) to form copper metal, aqueous. — in this. Copper Ii Chloride And Aluminum Equation.