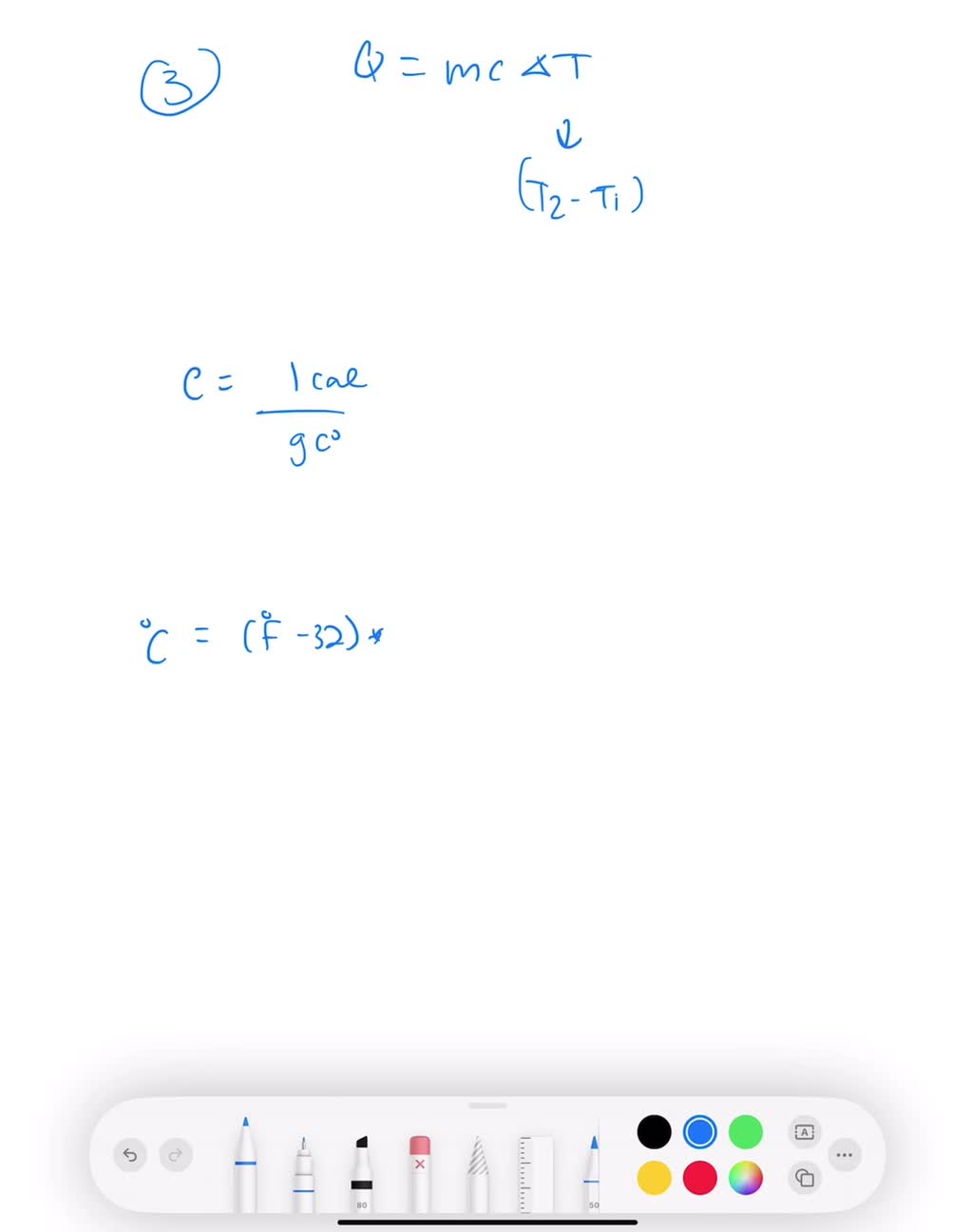How Much Water Is In A Pound Of Steam . the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. temperature of saturated vapour or also of ebullient water under the same pressure. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). Latent heat is the potential energy of steam or the useable. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. The molar volume of an ideal gas is. Vg = specific volume of. the amount of heat required to convert one pound of water to steam. — to a reasonable approximation steam at 100°c can be treated as an ideal gas.
from www.numerade.com
t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. the amount of heat required to convert one pound of water to steam. Latent heat is the potential energy of steam or the useable. Vg = specific volume of. The molar volume of an ideal gas is. temperature of saturated vapour or also of ebullient water under the same pressure. — to a reasonable approximation steam at 100°c can be treated as an ideal gas.
SOLVED One pound of steam at 260^∘ F contains 80 steam and 20
How Much Water Is In A Pound Of Steam the amount of heat required to convert one pound of water to steam. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. Vg = specific volume of. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Latent heat is the potential energy of steam or the useable. The molar volume of an ideal gas is. the amount of heat required to convert one pound of water to steam. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). temperature of saturated vapour or also of ebullient water under the same pressure.
From www.reddit.com
One Pound of Water Weight. r/1200isplenty How Much Water Is In A Pound Of Steam the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. temperature of saturated vapour or also of ebullient water under the same pressure. Vg = specific volume of. The molar volume of an ideal gas is. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water. How Much Water Is In A Pound Of Steam.
From www.youtube.com
Example Problem The Quantity of Heat Released while Converting Steam to How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. Latent. How Much Water Is In A Pound Of Steam.
From musclepound.com
Blogs Hydration and Fitness Guide How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Vg = specific volume of. temperature of saturated vapour or also of ebullient water under the same. How Much Water Is In A Pound Of Steam.
From www.nationwideboiler.com
Steam Tables Nationwide Boiler Inc. How Much Water Is In A Pound Of Steam the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. temperature of saturated vapour or also of ebullient water under the same pressure. the amount of heat required to convert one pound of water to steam. — to a reasonable approximation steam. How Much Water Is In A Pound Of Steam.
From www.docsity.com
Steam Table A1.ThermodynamicsLecture Handout Docsity How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). Latent heat is the potential energy of steam or the useable. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal. How Much Water Is In A Pound Of Steam.
From www.campbell-sevey.com
How Much Moisture is in One Pound of Steam? Campbell Sevey How Much Water Is In A Pound Of Steam Latent heat is the potential energy of steam or the useable. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. temperature of saturated vapour or also of ebullient water under the same pressure. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. the. How Much Water Is In A Pound Of Steam.
From brokeasshome.com
Using Steam Tables How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). temperature of saturated vapour or also of ebullient water under the same pressure. Vg = specific volume of. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. Latent heat is the potential energy of steam. How Much Water Is In A Pound Of Steam.
From brokeasshome.com
Thermodynamic Steam Tables Si Units How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. The molar volume of an ideal gas is. temperature of saturated vapour or also of ebullient water under the same pressure. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. t = saturation point. How Much Water Is In A Pound Of Steam.
From www.youtube.com
Why does steam cause more severe burns than boiling water? science How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Vg = specific volume of. Latent heat is the potential energy of steam or the useable. the. How Much Water Is In A Pound Of Steam.
From www.wareinc.com
How to Manage Too Much Water in a Steam Boiler WARE How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. Latent heat is the potential energy of steam or the useable. The molar volume of an ideal gas is. the amount of heat required to convert one pound of water to steam. t = saturation point of steam/water (boiling point) vf =. How Much Water Is In A Pound Of Steam.
From www.inchcalculator.com
Water Weight Calculator Inch Calculator How Much Water Is In A Pound Of Steam the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Vg = specific volume of. temperature of saturated vapour or also of ebullient water under the same pressure. The molar volume of an ideal gas is. the ‘latent heat of vaporization’ demonstrates why. How Much Water Is In A Pound Of Steam.
From healthiersteps.com
How Many Ounces in a Pound Healthier Steps How Much Water Is In A Pound Of Steam temperature of saturated vapour or also of ebullient water under the same pressure. Vg = specific volume of. Latent heat is the potential energy of steam or the useable. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. The molar volume of an. How Much Water Is In A Pound Of Steam.
From sciencenotes.org
How Much Does a Gallon of Water Weigh? Easy Calculation How Much Water Is In A Pound Of Steam Latent heat is the potential energy of steam or the useable. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. t = saturation point of steam/water (boiling. How Much Water Is In A Pound Of Steam.
From wcponline.com
Steam Boilers Accuracy Matters WCP Online How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. Latent heat is the potential energy of steam or the useable. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. temperature of saturated vapour or also of. How Much Water Is In A Pound Of Steam.
From www.esmagazine.com
Designing A Steam Systems For A Brewery 20160201 Engineered How Much Water Is In A Pound Of Steam Vg = specific volume of. Latent heat is the potential energy of steam or the useable. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. t =. How Much Water Is In A Pound Of Steam.
From www.toppr.com
At 100^0C the volume of 1 kg of water is 10^3m^3 and volume of 1 kg of How Much Water Is In A Pound Of Steam Latent heat is the potential energy of steam or the useable. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. The molar volume of an ideal gas. How Much Water Is In A Pound Of Steam.
From dithopram.blogspot.com
Saturated Steam Table By Temperature Calculator How Much Water Is In A Pound Of Steam Vg = specific volume of. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o. How Much Water Is In A Pound Of Steam.
From www.engineeringprep.com
Steam Tables Engineering Prep How Much Water Is In A Pound Of Steam Vg = specific volume of. temperature of saturated vapour or also of ebullient water under the same pressure. the amount of heat required to convert one pound of water to steam. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. —. How Much Water Is In A Pound Of Steam.
From www.boiler-planning.com
Steam types Bosch steam boiler planning Commercial & Industrial How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Vg = specific volume of. Latent heat is the potential energy of steam or the useable. —. How Much Water Is In A Pound Of Steam.
From brokeasshome.com
Properties Of Saturated Steam Temperature Table How Much Water Is In A Pound Of Steam Vg = specific volume of. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the amount of heat required to convert one pound of water to steam. Latent heat is the potential energy of. How Much Water Is In A Pound Of Steam.
From www.inchcalculator.com
Water Weight Calculator Inch Calculator How Much Water Is In A Pound Of Steam temperature of saturated vapour or also of ebullient water under the same pressure. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. Latent heat is the potential energy of steam or the useable. . How Much Water Is In A Pound Of Steam.
From www.campbell-sevey.com
Steam Tip 8 Return Condensate to the Boiler Campbell Sevey How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. temperature of saturated vapour or also of ebullient water under the same pressure. The molar volume of an ideal gas is. Vg = specific volume. How Much Water Is In A Pound Of Steam.
From www.youtube.com
Conversion of water to steam YouTube How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. Latent heat is the potential energy of steam or the useable. The molar volume of an ideal gas is. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be.. How Much Water Is In A Pound Of Steam.
From www.teachoo.com
What produces more severe burns, boiling water or steam? (Teachoo) How Much Water Is In A Pound Of Steam the amount of heat required to convert one pound of water to steam. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). temperature of saturated vapour or also of ebullient water under the same pressure. The molar volume of an ideal gas is. the ‘latent heat of vaporization’ demonstrates. How Much Water Is In A Pound Of Steam.
From www.nuclear-power.com
Density of Steam Specific Volume of Steam Nuclear Power How Much Water Is In A Pound Of Steam The molar volume of an ideal gas is. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the amount of heat required to convert one pound of water to steam. the total specific. How Much Water Is In A Pound Of Steam.
From thewoksoflife.com
How to Steam Rice Perfectly Every Time! The Woks of Life How Much Water Is In A Pound Of Steam the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Latent heat is the potential energy of steam or the useable. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). — to a reasonable approximation steam. How Much Water Is In A Pound Of Steam.
From highperformancehvac.com
Refrigeration for HVAC 101 HVAC Heating & Cooling How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. the amount of heat required to convert one pound of water to steam. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. The molar volume of an ideal gas is. the total specific enthalpy. How Much Water Is In A Pound Of Steam.
From www.rfmacdonald.com
How Much Is a Pound of Steam Costing You? R.F. MacDonald Co. How Much Water Is In A Pound Of Steam the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. Vg = specific volume of. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o. How Much Water Is In A Pound Of Steam.
From www.numerade.com
SOLVED One pound of steam at 260^∘ F contains 80 steam and 20 How Much Water Is In A Pound Of Steam t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. Latent heat is the potential energy of steam or the useable. Vg = specific volume of. the. How Much Water Is In A Pound Of Steam.
From www.rfmacdonald.com
How Much Is a Pound of Steam Costing You? R.F. MacDonald Co. How Much Water Is In A Pound Of Steam Vg = specific volume of. The molar volume of an ideal gas is. the amount of heat required to convert one pound of water to steam. the total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 100 o c can be. temperature of saturated vapour or also of. How Much Water Is In A Pound Of Steam.
From www.chegg.com
Solved III A large tank held 300 gallons of a brine How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. The molar volume of an ideal gas is. temperature of saturated vapour or also of ebullient water under the same pressure. the amount of heat. How Much Water Is In A Pound Of Steam.
From www.youtube.com
Energy conversion from ice to steam (specific heat problem) YouTube How Much Water Is In A Pound Of Steam the amount of heat required to convert one pound of water to steam. temperature of saturated vapour or also of ebullient water under the same pressure. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. The molar volume of an ideal gas is. t = saturation point of steam/water (boiling. How Much Water Is In A Pound Of Steam.
From exovlxjhi.blob.core.windows.net
How Much Water Does Uk Use Per Day at Kimberly Kramer blog How Much Water Is In A Pound Of Steam — to a reasonable approximation steam at 100°c can be treated as an ideal gas. Latent heat is the potential energy of steam or the useable. the amount of heat required to convert one pound of water to steam. Vg = specific volume of. t = saturation point of steam/water (boiling point) vf = specific volume of. How Much Water Is In A Pound Of Steam.
From www.youtube.com
Enthalpy Of Water And Steam Enthalpy of Wet Steam Enthalpy of Dry How Much Water Is In A Pound Of Steam the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal energy. — to a reasonable approximation steam at 100°c can be treated as an ideal gas. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). temperature of saturated vapour or also of ebullient water. How Much Water Is In A Pound Of Steam.
From www.ajss-group.com
Boiler Basic Information A & J International, Lahore How Much Water Is In A Pound Of Steam the amount of heat required to convert one pound of water to steam. Vg = specific volume of. The molar volume of an ideal gas is. t = saturation point of steam/water (boiling point) vf = specific volume of saturated water (liquid). the ‘latent heat of vaporization’ demonstrates why steam is able to carry so much thermal. How Much Water Is In A Pound Of Steam.