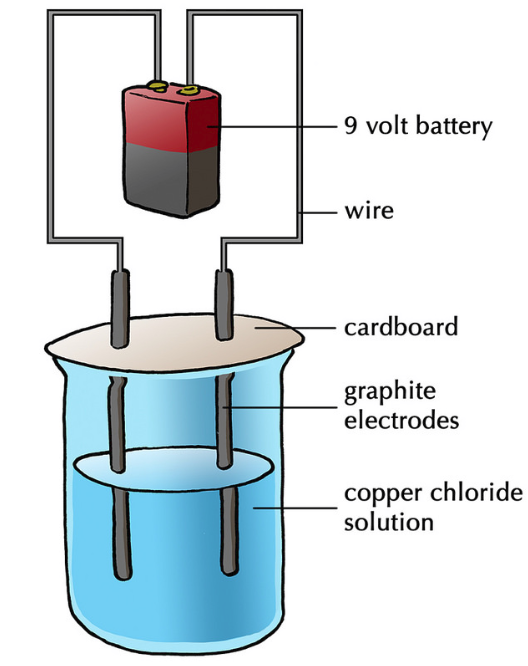
The Electrodes Used In Electrolysis . Reactive metals are extracted from their ores using electrolysis. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. By exploiting the cheapest and greenest source of. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Electrolysis can also be used to produce h. what are electrolytes and what happens in electrolysis? the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. organic electrosynthesis is an old and rich discipline.
from keystagewiki.com
what are electrolytes and what happens in electrolysis? By exploiting the cheapest and greenest source of. organic electrosynthesis is an old and rich discipline. Electrolysis can also be used to produce h. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. Reactive metals are extracted from their ores using electrolysis. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the.
Electrolysis Key Stage Wiki
The Electrodes Used In Electrolysis in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. Electrolysis can also be used to produce h. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. By exploiting the cheapest and greenest source of. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. what are electrolytes and what happens in electrolysis? in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. organic electrosynthesis is an old and rich discipline. Reactive metals are extracted from their ores using electrolysis.
From www.mbrashem.com
Why Are Graphite Electrodes Used in Electrolysis? M. Brashem, Inc The Electrodes Used In Electrolysis organic electrosynthesis is an old and rich discipline. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency. The Electrodes Used In Electrolysis.
From www.doubtnut.com
electrodes are used during electrolysis of acidulated wate The Electrodes Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. By exploiting the cheapest and greenest source of. Reactive metals are extracted from their ores using electrolysis. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of. The Electrodes Used In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk The Electrodes Used In Electrolysis the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. Electrolysis can also be used to produce h. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. An ionic compound occurs when a negative ion (an atom that has gained an electron). The Electrodes Used In Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS The Electrodes Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. Reactive metals are extracted from their ores using electrolysis. By exploiting the cheapest and greenest source. The Electrodes Used In Electrolysis.
From www.snexplores.org
Explainer What is an electrode? The Electrodes Used In Electrolysis An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. what are electrolytes and what happens in electrolysis? in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the choice of electrodes used in electrolysis plays a crucial role. The Electrodes Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 The Electrodes Used In Electrolysis By exploiting the cheapest and greenest source of. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. organic electrosynthesis is an old and rich discipline. what are electrolytes and what happens in. The Electrodes Used In Electrolysis.
From philschatz.com
Electrolysis · Chemistry The Electrodes Used In Electrolysis the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. Electrolysis can also be used to produce h. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. By exploiting the cheapest and greenest source of. this chapter provides a broad introduction to electrolysis and the. The Electrodes Used In Electrolysis.
From www.pinterest.com
What is Electrolytic cell Definition,Construction and working of The Electrodes Used In Electrolysis Reactive metals are extracted from their ores using electrolysis. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. organic electrosynthesis is an old and rich discipline. what are electrolytes and what happens in electrolysis? in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. By exploiting the. The Electrodes Used In Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps The Electrodes Used In Electrolysis An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this chapter provides a broad introduction to. The Electrodes Used In Electrolysis.
From 2012books.lardbucket.org
Electrolysis The Electrodes Used In Electrolysis the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. what are electrolytes and what happens in electrolysis?. The Electrodes Used In Electrolysis.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki The Electrodes Used In Electrolysis the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. Electrolysis can also be used to produce h. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive. The Electrodes Used In Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of copper sulphate using copper electrodes The Electrodes Used In Electrolysis organic electrosynthesis is an old and rich discipline. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. in any electrochemical cell (electrolytic or galvanic) the electrode at. The Electrodes Used In Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog The Electrodes Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Electrolysis can also be used to produce h. organic electrosynthesis is an old and rich discipline. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. . The Electrodes Used In Electrolysis.
From www.youtube.com
Physics Experiment Electrolysis Of Water With Zinc Electrodes Full HD The Electrodes Used In Electrolysis An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used. The Electrodes Used In Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki The Electrodes Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. organic electrosynthesis is an old and rich discipline. the choice of electrodes used in electrolysis plays a crucial role in determining. The Electrodes Used In Electrolysis.
From circuitcoffeegirl89t1.z14.web.core.windows.net
What Forms At The Cathode During Electrolysis The Electrodes Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs. The Electrodes Used In Electrolysis.
From saylordotorg.github.io
Electrolysis The Electrodes Used In Electrolysis Reactive metals are extracted from their ores using electrolysis. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in electrolysis, an external voltage is. The Electrodes Used In Electrolysis.
From www.alamy.com
Electrolysis of copper sulphate solution using copper electrodes Stock The Electrodes Used In Electrolysis An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. Reactive metals are extracted from their ores using electrolysis. Electrolysis can also be used to produce h. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. electrolysis can. The Electrodes Used In Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry Atoms First The Electrodes Used In Electrolysis Electrolysis can also be used to produce h. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. what are electrolytes and what happens in electrolysis? By exploiting the cheapest and greenest source of. organic electrosynthesis is an old and rich discipline. electrolysis can occur in electrolytic cells by introducing. The Electrodes Used In Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace The Electrodes Used In Electrolysis organic electrosynthesis is an old and rich discipline. Electrolysis can also be used to produce h. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. what are electrolytes and what happens in electrolysis? An ionic compound. The Electrodes Used In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download The Electrodes Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. By exploiting the cheapest and greenest source of. what are electrolytes and what happens in electrolysis? in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. An ionic compound occurs when a. The Electrodes Used In Electrolysis.
From keystagewiki.com
Electrolysis Key Stage Wiki The Electrodes Used In Electrolysis Reactive metals are extracted from their ores using electrolysis. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Electrolysis can also be used to produce h. organic electrosynthesis is an old and rich discipline. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to. The Electrodes Used In Electrolysis.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry The Electrodes Used In Electrolysis in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. By exploiting the cheapest and greenest source of. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Reactive metals are extracted from their ores using electrolysis. what. The Electrodes Used In Electrolysis.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse The Electrodes Used In Electrolysis Electrolysis can also be used to produce h. what are electrolytes and what happens in electrolysis? Reactive metals are extracted from their ores using electrolysis. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. An ionic compound occurs. The Electrodes Used In Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo The Electrodes Used In Electrolysis in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. what are electrolytes and what happens in electrolysis? organic electrosynthesis is an old and rich discipline. By exploiting the cheapest and greenest source of. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. An. The Electrodes Used In Electrolysis.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman The Electrodes Used In Electrolysis Electrolysis can also be used to produce h. organic electrosynthesis is an old and rich discipline. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. this chapter provides. The Electrodes Used In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 The Electrodes Used In Electrolysis in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. By exploiting the cheapest and greenest source of. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies. The Electrodes Used In Electrolysis.
From exowuasqn.blob.core.windows.net
Stainless Steel Electrodes For Electrolysis at Loretta Bryan blog The Electrodes Used In Electrolysis Electrolysis can also be used to produce h. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the choice of electrodes used in electrolysis plays a crucial role in determining the. The Electrodes Used In Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of acidified water with Platinum electrodes The Electrodes Used In Electrolysis in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. Electrolysis can also be used to produce h. organic electrosynthesis is an old and rich discipline. what are electrolytes and what happens in. The Electrodes Used In Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry The Electrodes Used In Electrolysis organic electrosynthesis is an old and rich discipline. Reactive metals are extracted from their ores using electrolysis. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. what are electrolytes and what happens in electrolysis?. The Electrodes Used In Electrolysis.
From courses.lumenlearning.com
17.2 Galvanic Cells Chemistry The Electrodes Used In Electrolysis in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. organic electrosynthesis is an old and rich discipline. By exploiting the cheapest and greenest source of. what. The Electrodes Used In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk The Electrodes Used In Electrolysis organic electrosynthesis is an old and rich discipline. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. Reactive metals are extracted from their ores using electrolysis. An ionic compound occurs when a negative. The Electrodes Used In Electrolysis.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram The Electrodes Used In Electrolysis organic electrosynthesis is an old and rich discipline. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. Electrolysis can also be used to produce h. in any electrochemical cell (electrolytic or galvanic) the electrode at which. The Electrodes Used In Electrolysis.
From blog.thepipingmart.com
Why are carbon electrodes used in electrolysis? The Electrodes Used In Electrolysis By exploiting the cheapest and greenest source of. An ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom. the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. in any electrochemical cell (electrolytic or galvanic) the electrode. The Electrodes Used In Electrolysis.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process The Electrodes Used In Electrolysis the choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the. this chapter provides a broad introduction to electrolysis and the use of electrolysers, using electricity via various. organic electrosynthesis is an old and rich discipline. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction.. The Electrodes Used In Electrolysis.