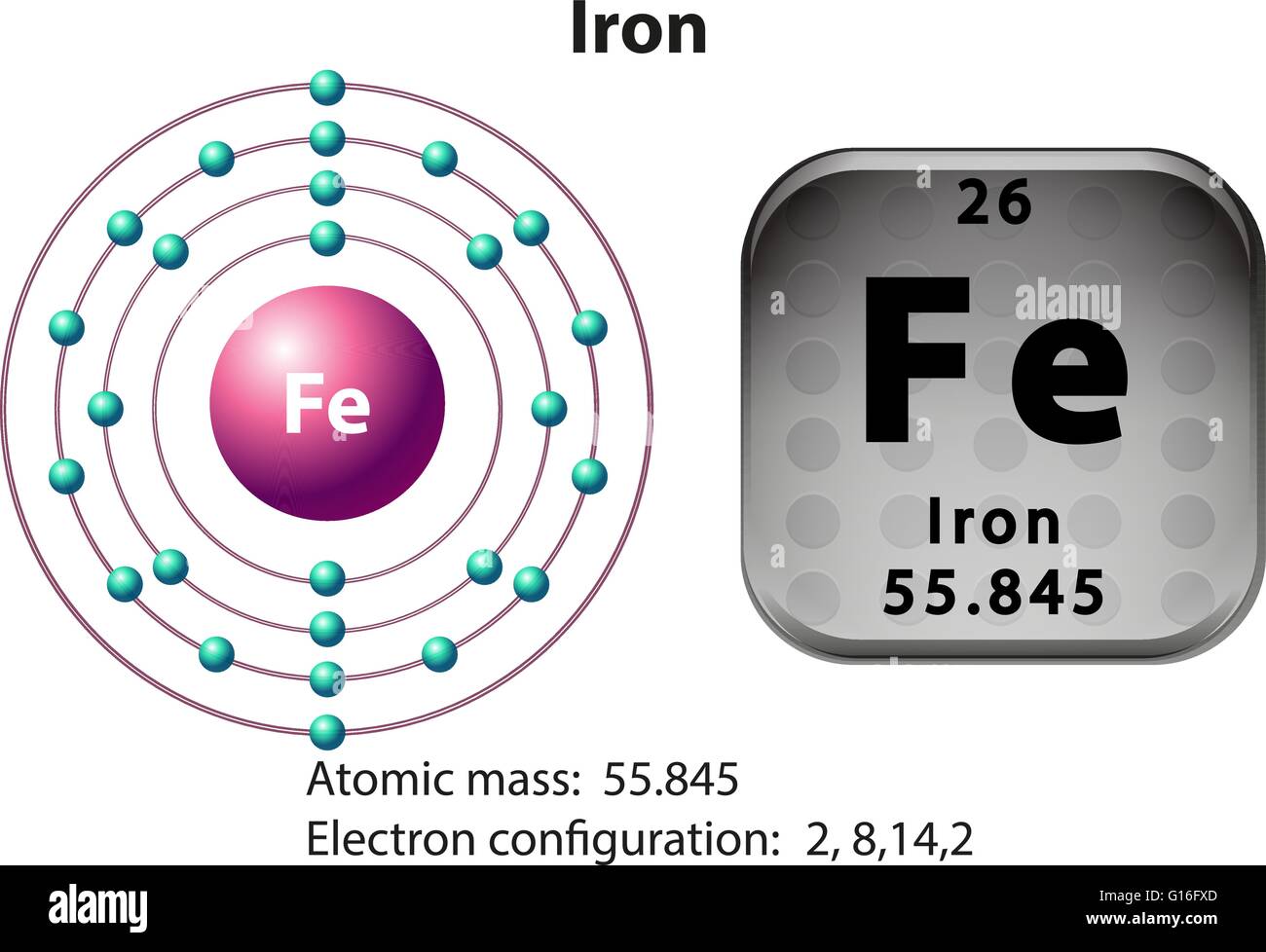
Iron Iii Atomic Mass . This compound is also known as ferric oxide or hematite or iron (iii) oxide. Count the number of each atom. Calculate molar mass of each element: It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Molar mass of iron(iii) = 602.93651 g/mol. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Molar mass of fe2o3 = 159.6882 g/mol. Multiply the atomic mass of each element by the number of atoms of that element in the compound. The atomic mass is usually found on the periodic. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Look up the atomic masses of each element present in the compound. Convert grams iron(iii) to moles.
from mungfali.com
Look up the atomic masses of each element present in the compound. Count the number of each atom. Molar mass of fe2o3 = 159.6882 g/mol. Multiply the atomic mass of each element by the number of atoms of that element in the compound. Convert grams iron(iii) to moles. Calculate molar mass of each element: Molar mass of iron(iii) = 602.93651 g/mol. This compound is also known as ferric oxide or hematite or iron (iii) oxide. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. The atomic mass is usually found on the periodic.
Chemical Structure Of Iron
Iron Iii Atomic Mass The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The atomic mass is usually found on the periodic. Molar mass of iron(iii) = 602.93651 g/mol. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Look up the atomic masses of each element present in the compound. Count the number of each atom. Convert grams iron(iii) to moles. Multiply the atomic mass of each element by the number of atoms of that element in the compound. Calculate molar mass of each element: Molar mass of fe2o3 = 159.6882 g/mol.
From molekula.com
Purchase Iron(III) chloride hexahydrate [10025771] online • Catalog Iron Iii Atomic Mass Convert grams iron(iii) to moles. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. This compound is also known as ferric oxide or hematite or iron (iii) oxide. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Multiply the atomic mass of each element by the number of atoms of. Iron Iii Atomic Mass.
From www.adda247.com
Atomic Mass of Elements 1 to 30 with Symbols PDF Download Iron Iii Atomic Mass Look up the atomic masses of each element present in the compound. Molar mass of iron(iii) = 602.93651 g/mol. The atomic mass is usually found on the periodic. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. It is soluble. Iron Iii Atomic Mass.
From alquilercastilloshinchables.info
8 Photos Iron Periodic Table And Description Alqu Blog Iron Iii Atomic Mass The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Calculate molar mass of each element: Count the number of each atom. Multiply the atomic mass of each element by the number of atoms of that element. Iron Iii Atomic Mass.
From www.slideserve.com
PPT Atomic Mass PowerPoint Presentation, free download ID3983503 Iron Iii Atomic Mass Multiply the atomic mass of each element by the number of atoms of that element in the compound. Molar mass of fe2o3 = 159.6882 g/mol. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Molar mass of iron(iii) = 602.93651 g/mol. Count the number of each atom. There are 4 easy steps to find the molar. Iron Iii Atomic Mass.
From www.toppr.com
Iron iii Sulfate Formula Structure, Preparations and Properties Iron Iii Atomic Mass It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Multiply the atomic mass of each element by the number of atoms of that element in the compound. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Molar mass of iron(iii) = 602.93651 g/mol. Molar mass of fe2o3 = 159.6882 g/mol.. Iron Iii Atomic Mass.
From www.slideserve.com
PPT Chemistry Mole Calculations The Mole & Molar Mass PowerPoint Iron Iii Atomic Mass Convert grams iron(iii) to moles. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The atomic mass is usually found on the. Iron Iii Atomic Mass.
From www.youtube.com
How to Calculate the Molar Mass / Molecular Weight of Fe2O3 Iron Iron Iii Atomic Mass Multiply the atomic mass of each element by the number of atoms of that element in the compound. Look up the atomic masses of each element present in the compound. Molar mass of iron(iii) = 602.93651 g/mol. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. The atomic mass is usually found on the periodic. Molar. Iron Iii Atomic Mass.
From www.vectorstock.com
Flashcard of iron with atomic mass Royalty Free Vector Image Iron Iii Atomic Mass The atomic mass is usually found on the periodic. Convert grams iron(iii) to moles. Calculate molar mass of each element: Molar mass of iron(iii) = 602.93651 g/mol. Count the number of each atom. Multiply the atomic mass of each element by the number of atoms of that element in the compound. The molar mass of iron(iii) oxide is approximately 159.7. Iron Iii Atomic Mass.
From www.numerade.com
SOLVED 4.0 g of iron(III) oxide (, molar mass = 160 g/mol) and 4.0 g Iron Iii Atomic Mass Convert grams iron(iii) to moles. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Molar mass of fe2o3 = 159.6882 g/mol. It. Iron Iii Atomic Mass.
From www.youtube.com
What is the molar mass of iron III oxide Fe2O3? YouTube Iron Iii Atomic Mass The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. The atomic mass is usually found on the periodic. Look up the atomic masses of each element present in the compound. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Calculate molar mass of each element:. Iron Iii Atomic Mass.
From www.numerade.com
What mass of iron(III) hydroxide precipitate can be produced by Iron Iii Atomic Mass Count the number of each atom. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Look up the atomic masses of each element present in the compound. Molar mass of fe2o3 = 159.6882 g/mol. Molar mass of. Iron Iii Atomic Mass.
From brainly.com
Calculator the mass of iron (III) oxide ( Fe2O3) that contains a Iron Iii Atomic Mass The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Molar mass of fe2o3 = 159.6882 g/mol. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Multiply the atomic mass of each element by the number of atoms of that element in the compound. Calculate molar. Iron Iii Atomic Mass.
From www.youtube.com
Molar Mass / Molecular Weight of Fe2(SO4)3 Iron (III) sulfate YouTube Iron Iii Atomic Mass There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. The atomic mass is usually found on the periodic. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Molar mass of iron(iii) = 602.93651 g/mol. Look up the atomic masses of each element present in the compound.. Iron Iii Atomic Mass.
From www.alamy.com
Iron chemical element. Chemical symbol with atomic number and atomic Iron Iii Atomic Mass Convert grams iron(iii) to moles. Molar mass of fe2o3 = 159.6882 g/mol. Calculate molar mass of each element: This compound is also known as ferric oxide or hematite or iron (iii) oxide. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Count the number of each atom. It is soluble in. Iron Iii Atomic Mass.
From www.youtube.com
How to Find the Average Atomic Mass of Iron YouTube Iron Iii Atomic Mass Molar mass of iron(iii) = 602.93651 g/mol. Convert grams iron(iii) to moles. Molar mass of fe2o3 = 159.6882 g/mol. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Look up the atomic masses of each element present in. Iron Iii Atomic Mass.
From www.numerade.com
SOLVED Calculate the mass of iron(III) oxide Fe2O3 that contains a Iron Iii Atomic Mass This compound is also known as ferric oxide or hematite or iron (iii) oxide. Molar mass of iron(iii) = 602.93651 g/mol. Calculate molar mass of each element: There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Molar mass of fe2o3 = 159.6882 g/mol. Count the number of each atom. It is. Iron Iii Atomic Mass.
From mungfali.com
Chemical Structure Of Iron Iron Iii Atomic Mass The atomic mass is usually found on the periodic. Multiply the atomic mass of each element by the number of atoms of that element in the compound. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Convert grams iron(iii) to moles. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water.. Iron Iii Atomic Mass.
From www.gauthmath.com
Solved You can use mole ratios and molar mass to determine the masses Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Count the number of each atom. Molar mass of iron(iii) = 602.93651 g/mol. Convert grams iron(iii) to moles. Look up the atomic masses of each element present in the compound. Calculate molar mass of each element: The atomic. Iron Iii Atomic Mass.
From www.periodictableprintable.com
Iron Periodic Table Electrons 2023 Periodic Table Printable Iron Iii Atomic Mass The atomic mass is usually found on the periodic. Molar mass of fe2o3 = 159.6882 g/mol. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Calculate molar mass of each element: Look up the atomic masses of each element present in the compound. The molar mass of iron(iii) oxide is approximately. Iron Iii Atomic Mass.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. The atomic mass is usually found on the periodic. Multiply the atomic mass of each element by the number of atoms of that element in the compound. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Convert grams iron(iii) to moles. Molar mass of iron(iii) = 602.93651. Iron Iii Atomic Mass.
From www.youtube.com
molar mass of Fe(OH)3 YouTube Iron Iii Atomic Mass Look up the atomic masses of each element present in the compound. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Molar mass of iron(iii) = 602.93651 g/mol. Molar mass of fe2o3 = 159.6882 g/mol. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The atomic. Iron Iii Atomic Mass.
From www.geeksforgeeks.org
Atomic Mass Table of First 30 Elements Iron Iii Atomic Mass Look up the atomic masses of each element present in the compound. The atomic mass is usually found on the periodic. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate molar mass of each element: Molar mass of iron(iii) = 602.93651 g/mol. Convert grams iron(iii) to moles. It is soluble in strong acids such. Iron Iii Atomic Mass.
From utedzz.blogspot.com
Periodic Table Iron Atomic Mass Periodic Table Timeline Iron Iii Atomic Mass Count the number of each atom. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Molar mass of fe2o3 = 159.6882 g/mol. Multiply the atomic mass of each element by the number of atoms of that. Iron Iii Atomic Mass.
From www.youtube.com
How to find the molar mass of Fe2(SO4)3 (Iron (III) Sulfate) YouTube Iron Iii Atomic Mass Convert grams iron(iii) to moles. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Multiply the atomic mass of each element by the number of atoms of that element in the compound. Molar mass of iron(iii) = 602.93651 g/mol. Count the number of each atom. Molar mass of fe2o3 = 159.6882. Iron Iii Atomic Mass.
From 139.59.164.119
Periodic Table with Atomic Mass Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Calculate molar mass of each element: Count the number of each atom. Molar mass of iron(iii) = 602.93651 g/mol. The atomic mass is usually found. Iron Iii Atomic Mass.
From www.chegg.com
Solved DEMONSTRATION iron(III) nitrate nonahydrate Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. Look up the atomic masses of each element present in the compound. Calculate molar mass of each element: This compound is also known as ferric oxide or hematite or iron (iii) oxide. The atomic mass is usually found on the periodic. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is. Iron Iii Atomic Mass.
From www.slideserve.com
PPT Stoichiometry PowerPoint Presentation, free download ID3919556 Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. Calculate molar mass of each element: Look up the atomic masses of each element present in the compound. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Molar mass of iron(iii) = 602.93651 g/mol. This compound is also known as ferric oxide or hematite or iron (iii) oxide. There. Iron Iii Atomic Mass.
From tankhrom.weebly.com
Iron atomic number tankhrom Iron Iii Atomic Mass There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Molar mass of fe2o3 = 159.6882 g/mol. Calculate molar mass of each element: Multiply the atomic mass of each element by the number of atoms of that element. Iron Iii Atomic Mass.
From ootery.weebly.com
Atomic mass of iron ootery Iron Iii Atomic Mass Look up the atomic masses of each element present in the compound. Molar mass of iron(iii) = 602.93651 g/mol. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. Count the number of each atom. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate molar mass. Iron Iii Atomic Mass.
From www.coursehero.com
[Solved] calculating the molar mass of the ionic compound iron(III Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Convert grams iron(iii) to moles. The atomic mass is usually found. Iron Iii Atomic Mass.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. There are 4 easy steps to find the molar mass of fe {3+} based on its chemical formula. The atomic mass is usually found on the periodic. Look up the atomic masses of each element present in the compound. Count the number of each atom. It is soluble in strong acids such as. Iron Iii Atomic Mass.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron Iii Atomic Mass This compound is also known as ferric oxide or hematite or iron (iii) oxide. Count the number of each atom. The atomic mass is usually found on the periodic. Multiply the atomic mass of each element by the number of atoms of that element in the compound. It is soluble in strong acids such as hydrochloric acid and sulfuric acid.. Iron Iii Atomic Mass.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Iron Iii Atomic Mass It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Convert grams iron(iii) to moles. Multiply the atomic mass of each element by the number of atoms of that element in the compound. Calculate molar mass of each element: Look up the atomic masses of each element present in the compound. There are 4 easy steps to. Iron Iii Atomic Mass.
From www.dreamstime.com
Round Periodic Table Element Symbol of Iron Stock Vector Illustration Iron Iii Atomic Mass Molar mass of fe2o3 = 159.6882 g/mol. It is soluble in strong acids such as hydrochloric acid and sulfuric acid. The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Calculate molar mass of each element: Multiply the atomic mass of each element by the number of atoms of that element in the compound. There. Iron Iii Atomic Mass.
From www.slideserve.com
PPT MOLE (mol) PowerPoint Presentation, free download ID4272649 Iron Iii Atomic Mass The atomic mass is usually found on the periodic. Count the number of each atom. Molar mass of fe2o3 = 159.6882 g/mol. Calculate molar mass of each element: Molar mass of iron(iii) = 602.93651 g/mol. This compound is also known as ferric oxide or hematite or iron (iii) oxide. There are 4 easy steps to find the molar mass of. Iron Iii Atomic Mass.