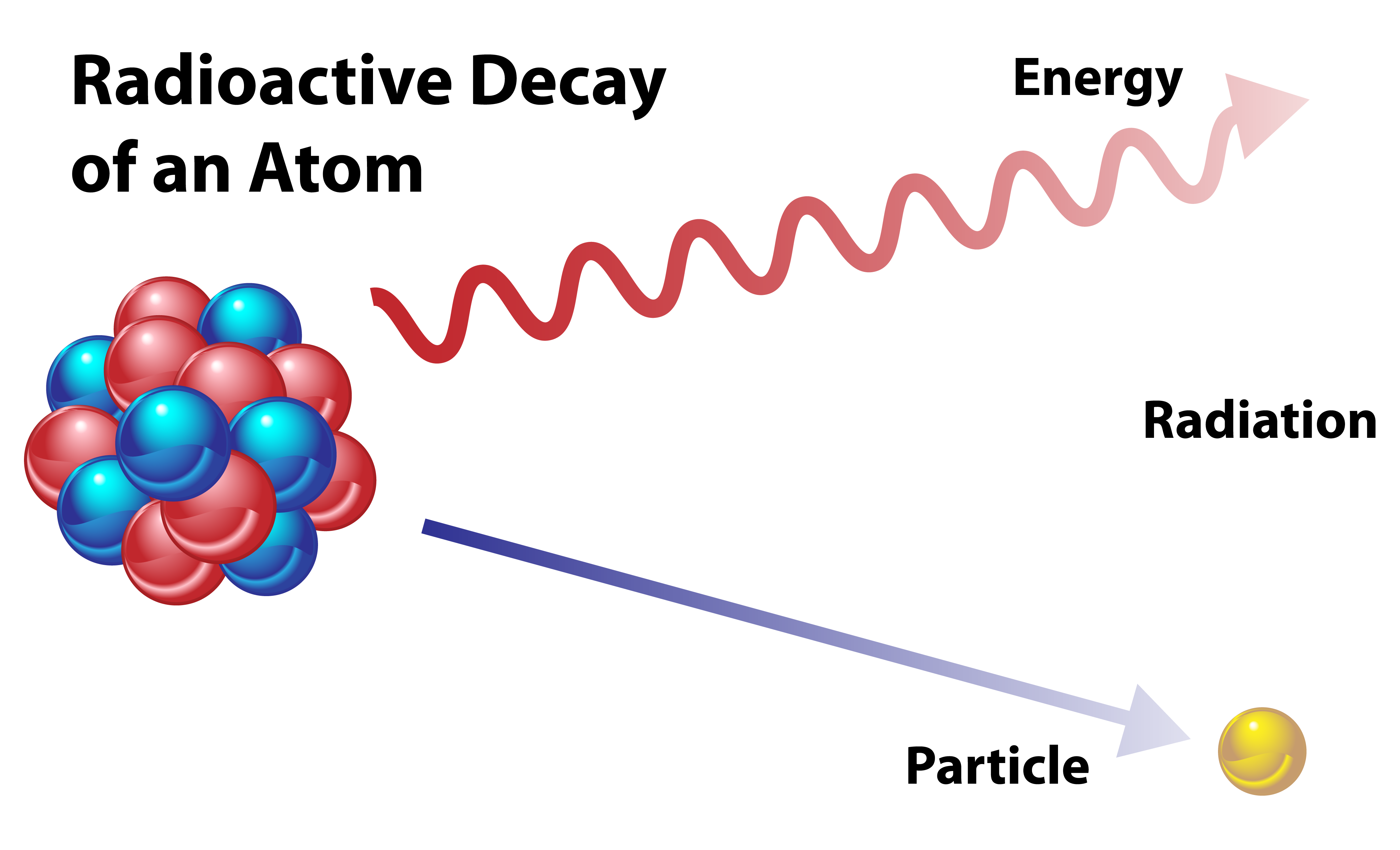
Radioactive Chemistry Definition . Explain how radioactivity involves a change in the nucleus of a radioisotope. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. This phenomenon is called radioactivity Thus all isotopes of all elements beyond bismuth in the. To reach a lower, more stable energy level, it releases energy in the form of radiation. The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha.
from www.yaclass.in
The nuclei of all elements with atomic numbers greater than 83 are unstable. To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. Explain how radioactivity involves a change in the nucleus of a radioisotope. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Thus all isotopes of all elements beyond bismuth in the.
Radiochemistry and its applications — lesson. Science State Board, Class 9.
Radioactive Chemistry Definition radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. The nuclei of all elements with atomic numbers greater than 83 are unstable. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. Thus all isotopes of all elements beyond bismuth in the. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. This phenomenon is called radioactivity To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. Explain how radioactivity involves a change in the nucleus of a radioisotope. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions.
From www.slideserve.com
PPT Radioactivity PowerPoint Presentation, free download ID296273 Radioactive Chemistry Definition radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. Thus all isotopes of all elements beyond bismuth in the. This phenomenon is called radioactivity The nuclei of all elements with atomic numbers greater than 83 are unstable. To reach a lower, more stable energy level, it releases. Radioactive Chemistry Definition.
From dxovhmyvz.blob.core.windows.net
Radioactive Definition Chemistry at Brett Ezzell blog Radioactive Chemistry Definition radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. Explain how radioactivity involves a change in the nucleus of a radioisotope. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity is the process by which the nucleus of an unstable. Radioactive Chemistry Definition.
From sciencestruck.com
List of Radioactive Elements Radioactive Chemistry Definition Explain how radioactivity involves a change in the nucleus of a radioisotope. Thus all isotopes of all elements beyond bismuth in the. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. To reach a lower, more stable energy level, it releases energy in the form of radiation. This radiation can be emitted as particles or. Radioactive Chemistry Definition.
From www.scienceabc.com
Why Are Certain Elements Radioactive? » ScienceABC Radioactive Chemistry Definition radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. radioactivity occurs when an atom has an excess of energy, mass, or both,. Radioactive Chemistry Definition.
From www.dreamstime.com
Radioactivity. Closeup of Radioactive Atom Stock Vector Illustration Radioactive Chemistry Definition radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity is the spontaneous emission of radiation in the form. Radioactive Chemistry Definition.
From www.pinterest.com
Types of radiation vector illustration diagram Physik und mathematik Radioactive Chemistry Definition This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. The nuclei of all elements with atomic numbers greater than 83 are unstable. Thus all isotopes of all elements beyond bismuth. Radioactive Chemistry Definition.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Radioactive Chemistry Definition Thus all isotopes of all elements beyond bismuth in the. Explain how radioactivity involves a change in the nucleus of a radioisotope. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the. Radioactive Chemistry Definition.
From www.thoughtco.com
A List of Radioactive Elements Radioactive Chemistry Definition This phenomenon is called radioactivity The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. radioactivity occurs when an atom has an excess. Radioactive Chemistry Definition.
From nfwfoundation.org
About radiation Nuclear Free World Foundation Radioactive Chemistry Definition radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Explain how radioactivity involves a change in the nucleus of a radioisotope. To reach a lower, more stable energy level, it releases energy in the form of radiation. This phenomenon is called radioactivity Thus all isotopes of all elements beyond bismuth in. Radioactive Chemistry Definition.
From www.geeksforgeeks.org
Radioactive Elements History, Examples, List, and Applications Radioactive Chemistry Definition radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactivity occurs when an atom has an excess of energy, mass, or both,. Radioactive Chemistry Definition.
From definitionkd.blogspot.com
Radioactive Decay Definition Chemistry DEFINITIONKD Radioactive Chemistry Definition The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. This phenomenon is called radioactivity radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. To reach. Radioactive Chemistry Definition.
From www.slideshare.net
Radioactive Elements Radioactive Chemistry Definition radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. To reach a lower, more stable energy level, it releases energy in the form of radiation. This phenomenon is called radioactivity radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. This radiation. Radioactive Chemistry Definition.
From courses.lumenlearning.com
Radioactive Decay Chemistry for Majors Radioactive Chemistry Definition This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. This phenomenon. Radioactive Chemistry Definition.
From www.vrogue.co
Radioactive Decay Chemistry Atoms First vrogue.co Radioactive Chemistry Definition The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. Thus all isotopes of all elements beyond bismuth in. Radioactive Chemistry Definition.
From www.epa.gov
Radiation Sources and Doses US EPA Radioactive Chemistry Definition radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from. Radioactive Chemistry Definition.
From facts.net
16 Captivating Facts About Radioactive Isotope Radioactive Chemistry Definition The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. To reach a lower, more stable energy level, it releases energy in the form of radiation. Explain how radioactivity involves a change in the nucleus of a radioisotope. This phenomenon is called radioactivity The nuclei of all elements. Radioactive Chemistry Definition.
From www.slideserve.com
PPT Radioactive Decays transmutations of nuclides PowerPoint Radioactive Chemistry Definition Thus all isotopes of all elements beyond bismuth in the. To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha.. Radioactive Chemistry Definition.
From gamesmartz.com
Radioactivity Definition & Image GameSmartz Radioactive Chemistry Definition radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear. Radioactive Chemistry Definition.
From exoeuspex.blob.core.windows.net
Radioactive Definition Biology at Brook Neal blog Radioactive Chemistry Definition radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. The nuclei of all elements with atomic numbers greater than 83 are unstable. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. To reach a lower, more. Radioactive Chemistry Definition.
From www.youtube.com
NUCLEAR CHEMISTRY Radioactivity & Radiation Alpha, Beta, Gamma Radioactive Chemistry Definition This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. This phenomenon is called radioactivity The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity occurs when an atom has an excess of energy, mass, or both, making its. Radioactive Chemistry Definition.
From dxovhmyvz.blob.core.windows.net
Radioactive Definition Chemistry at Brett Ezzell blog Radioactive Chemistry Definition radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. This phenomenon is called radioactivity The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. This radiation can be emitted as particles or electromagnetic waves, depending. Radioactive Chemistry Definition.
From rorynewscharles.blogspot.com
Describe Three Uses of Radioactive Elements Radioactive Chemistry Definition radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. This phenomenon is called radioactivity radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. Thus all isotopes of all. Radioactive Chemistry Definition.
From gamesmartz.com
Radioactive Decay Definition & Image GameSmartz Radioactive Chemistry Definition radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. To reach a lower, more stable energy level, it releases energy in the form of radiation. This phenomenon is called radioactivity Thus all isotopes of all elements beyond bismuth in the. Explain how radioactivity involves a change in the nucleus of a. Radioactive Chemistry Definition.
From exouwqqfz.blob.core.windows.net
What Is A Radioactive Test at Glen Rogers blog Radioactive Chemistry Definition radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. This phenomenon is called radioactivity radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. Explain how radioactivity involves a change in the nucleus of a radioisotope. The. Radioactive Chemistry Definition.
From sciencenotes.org
What Are the Radioactive Elements? Radioactive Chemistry Definition radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. This phenomenon is called radioactivity Explain how radioactivity involves a change in the nucleus of a radioisotope. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. radioactivity is the. Radioactive Chemistry Definition.
From dxohcbbxy.blob.core.windows.net
What Is The Importance Of Radioactive Decay at Vicki Penniman blog Radioactive Chemistry Definition This phenomenon is called radioactivity radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Explain how radioactivity involves a change in the nucleus of a radioisotope. radioactivity occurs when an atom has. Radioactive Chemistry Definition.
From www.youtube.com
Uses of radioactive isotopes Chemistry YouTube Radioactive Chemistry Definition The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. radioactivity. Radioactive Chemistry Definition.
From www.slideserve.com
PPT Chemistry 142 Chapter 19 Radioactivity and Nuclear Chemistry Radioactive Chemistry Definition This phenomenon is called radioactivity This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. radioactivity occurs. Radioactive Chemistry Definition.
From www.slideserve.com
PPT Chapter 4 Nuclear Chemistry PowerPoint Presentation, free Radioactive Chemistry Definition radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic. Radioactive Chemistry Definition.
From sciencenotes.org
Radioactivity and the Types of Radioactive Decay Radioactive Chemistry Definition The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles. The three main types of radioactive decay are alpha, beta,. Radioactive Chemistry Definition.
From www.snexplores.org
Explainer Radiation and radioactive decay Radioactive Chemistry Definition This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. This phenomenon is called radioactivity radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha.. Radioactive Chemistry Definition.
From www.thoughtco.com
A List of Radioactive Elements Radioactive Chemistry Definition The nuclei of all elements with atomic numbers greater than 83 are unstable. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Thus all isotopes of all elements beyond bismuth in the. radioactive decay is a nucleus' journey to attaining stability via emission of highly energetic radiation and subatomic particles.. Radioactive Chemistry Definition.
From www.yaclass.in
Radiochemistry and its applications — lesson. Science State Board, Class 9. Radioactive Chemistry Definition The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Thus all isotopes of all elements. Radioactive Chemistry Definition.
From shakerscience.weebly.com
Unit 11 Nuclear Chemistry Physical Science Radioactive Chemistry Definition radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. This phenomenon is called radioactivity radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactive decay is a nucleus'. Radioactive Chemistry Definition.
From www.tes.com
Radioactive elements (Chemistry) Teaching Resources Radioactive Chemistry Definition radioactivity is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha. To reach a lower, more stable energy level, it releases energy in the form of radiation. This phenomenon is called radioactivity The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions. Radioactive Chemistry Definition.