Lead And Steel Reaction . galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. there are several factors affecting the rate of galvanic corrosion: The anodic metal will preferentially corrode. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. This chart is designed to assist in broadly assessing the risk of. below is a galvanic reaction chart for dissimilar metals. — the potential difference between the metals is the driving force behind the corrosive reaction. (1) the degree of potential difference between the two.
from www.onlinemathlearning.com
— galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. — the potential difference between the metals is the driving force behind the corrosive reaction. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. The anodic metal will preferentially corrode. (1) the degree of potential difference between the two. there are several factors affecting the rate of galvanic corrosion: This chart is designed to assist in broadly assessing the risk of. below is a galvanic reaction chart for dissimilar metals.
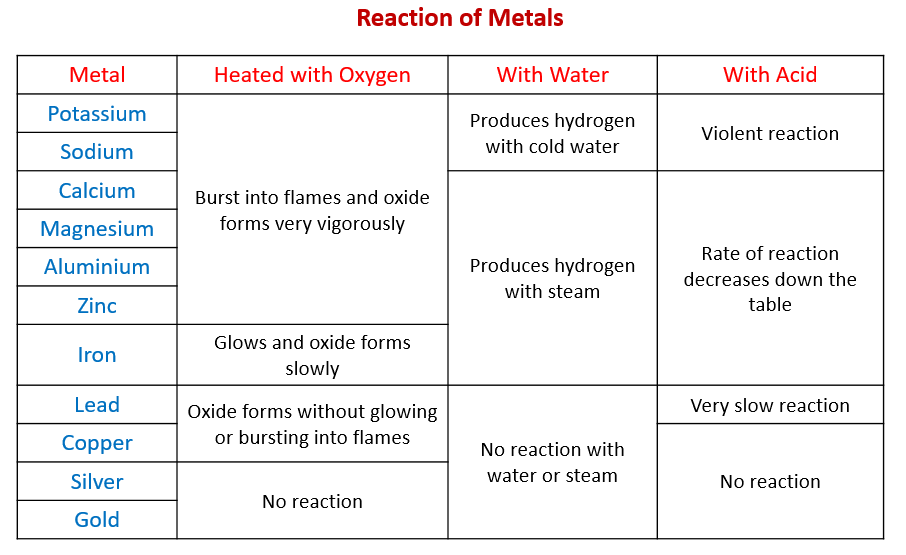
Reaction of Metals (examples, answers, activities, experiment, videos)
Lead And Steel Reaction The anodic metal will preferentially corrode. This chart is designed to assist in broadly assessing the risk of. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. below is a galvanic reaction chart for dissimilar metals. — the potential difference between the metals is the driving force behind the corrosive reaction. there are several factors affecting the rate of galvanic corrosion: — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. The anodic metal will preferentially corrode. (1) the degree of potential difference between the two.
From mrcartlidgesaigon.edublogs.org
C10 Metals Science) Mr Cartlidge's second Science Blog Lead And Steel Reaction (1) the degree of potential difference between the two. — the potential difference between the metals is the driving force behind the corrosive reaction. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. The anodic metal will preferentially corrode. below is a galvanic reaction chart for dissimilar. Lead And Steel Reaction.
From www.onlinemathlearning.com
Reaction of Metals (examples, answers, activities, experiment, videos) Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. (1) the degree of potential difference between the two. there are several factors affecting the rate of galvanic corrosion: below is a galvanic reaction chart for dissimilar metals. — the potential. Lead And Steel Reaction.
From www.youtube.com
Acid Metal Reactions YouTube Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. The anodic metal will preferentially corrode. This chart is designed to assist in broadly. Lead And Steel Reaction.
From www.slideserve.com
PPT Metal Displacement Reactions. PowerPoint Presentation, free Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. there are several factors affecting the rate of galvanic corrosion: (1) the degree of potential difference between the two. — the potential difference between the metals is the driving force behind the. Lead And Steel Reaction.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Lead And Steel Reaction (1) the degree of potential difference between the two. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — the potential difference between the metals is the driving force behind the corrosive reaction. This chart is designed to assist in broadly assessing the risk of. — galvanic. Lead And Steel Reaction.
From hinahanap6dschematic.z21.web.core.windows.net
What Forms At The Cathode During Electrolysis Lead And Steel Reaction below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. there are several factors affecting the rate of galvanic corrosion: . Lead And Steel Reaction.
From www.chemistrystudent.com
Group 2 Metal Reactions (ALevel) ChemistryStudent Lead And Steel Reaction below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of. (1) the degree of potential difference between the two. there are several factors affecting the rate of galvanic corrosion: The anodic metal will preferentially corrode. — the potential difference between the metals is the driving force. Lead And Steel Reaction.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Lead And Steel Reaction This chart is designed to assist in broadly assessing the risk of. below is a galvanic reaction chart for dissimilar metals. (1) the degree of potential difference between the two. The anodic metal will preferentially corrode. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also. Lead And Steel Reaction.
From exohiwrsf.blob.core.windows.net
Does Stainless Steel Have Lead In It at Dustin Black blog Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. The anodic metal will preferentially corrode. — the potential difference between the metals is the driving force behind the corrosive reaction. there are several factors affecting the rate of galvanic corrosion: below is a galvanic reaction chart. Lead And Steel Reaction.
From diagramdatareturfed.z22.web.core.windows.net
What Is Eutectic Point In Phase Diagrams Lead And Steel Reaction below is a galvanic reaction chart for dissimilar metals. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. This chart is designed. Lead And Steel Reaction.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Lead And Steel Reaction — the potential difference between the metals is the driving force behind the corrosive reaction. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. (1) the degree of potential difference between the two. This chart is designed to assist in broadly assessing the risk of. there are. Lead And Steel Reaction.
From recipepes.com
zinc and stainless steel reaction Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. — the potential difference between the metals is the driving force behind the corrosive reaction. The anodic metal will preferentially corrode. galvanic corrosion (some times called dissimilar metal corrosion) is the process. Lead And Steel Reaction.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. This chart is designed to assist in broadly assessing the risk of. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. below. Lead And Steel Reaction.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo Lead And Steel Reaction — the potential difference between the metals is the driving force behind the corrosive reaction. The anodic metal will preferentially corrode. below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials. Lead And Steel Reaction.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Lead And Steel Reaction below is a galvanic reaction chart for dissimilar metals. (1) the degree of potential difference between the two. This chart is designed to assist in broadly assessing the risk of. The anodic metal will preferentially corrode. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — the. Lead And Steel Reaction.
From www.quelpr.com
CSEC Chemistry Reactivity of Metals Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — the potential difference between the metals is the driving force behind the corrosive reaction. (1) the degree of potential difference between the two. there are several factors affecting the rate of galvanic corrosion: The anodic metal will. Lead And Steel Reaction.
From www.slideshare.net
Reaction Of Metal Oxides With Acid Lead And Steel Reaction The anodic metal will preferentially corrode. This chart is designed to assist in broadly assessing the risk of. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — the potential difference between the metals is the driving force behind the corrosive reaction. there are several factors affecting. Lead And Steel Reaction.
From www.alamy.com
Reactivity of metals. Magnesium, zinc, iron and lead in dilute Stock Lead And Steel Reaction This chart is designed to assist in broadly assessing the risk of. (1) the degree of potential difference between the two. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. The anodic metal will preferentially corrode. below is a galvanic reaction chart. Lead And Steel Reaction.
From www.youtube.com
Reactions between Metals and Acids YouTube Lead And Steel Reaction there are several factors affecting the rate of galvanic corrosion: below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — the potential difference between the. Lead And Steel Reaction.
From www.youtube.com
Reaction of Metals with Water 10th Std Chemistry ICSE Board Lead And Steel Reaction The anodic metal will preferentially corrode. there are several factors affecting the rate of galvanic corrosion: — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. (1) the degree of potential difference between the two. below is a galvanic reaction chart for. Lead And Steel Reaction.
From recipepes.com
zinc and stainless steel reaction Lead And Steel Reaction The anodic metal will preferentially corrode. This chart is designed to assist in broadly assessing the risk of. (1) the degree of potential difference between the two. there are several factors affecting the rate of galvanic corrosion: — the potential difference between the metals is the driving force behind the corrosive reaction. below is a galvanic reaction. Lead And Steel Reaction.
From blog.thepipingmart.com
StressStrain Curve for Mild Steel An Overview Lead And Steel Reaction — the potential difference between the metals is the driving force behind the corrosive reaction. This chart is designed to assist in broadly assessing the risk of. (1) the degree of potential difference between the two. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also. Lead And Steel Reaction.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Lead And Steel Reaction The anodic metal will preferentially corrode. — the potential difference between the metals is the driving force behind the corrosive reaction. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. (1) the degree of potential difference between the two. galvanic corrosion. Lead And Steel Reaction.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Lead And Steel Reaction This chart is designed to assist in broadly assessing the risk of. below is a galvanic reaction chart for dissimilar metals. (1) the degree of potential difference between the two. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — galvanic corrosion is an expensive issue which. Lead And Steel Reaction.
From chem.libretexts.org
23.3 Metallurgy of Iron and Steel Chemistry LibreTexts Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. there are several factors affecting the rate of galvanic corrosion: (1) the degree. Lead And Steel Reaction.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. This chart is designed to assist in broadly assessing the risk of. (1) the degree of potential difference between the two. — the potential difference between the metals is the driving force behind. Lead And Steel Reaction.
From projectupland.com
How Effective is Steel Shot? Comparing Lead to Steel Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. there are several factors affecting the rate of galvanic corrosion: The anodic metal. Lead And Steel Reaction.
From chemnotcheem.com
Redox reactions Oxidation and reduction O Level Chemistry Notes Lead And Steel Reaction This chart is designed to assist in broadly assessing the risk of. — the potential difference between the metals is the driving force behind the corrosive reaction. (1) the degree of potential difference between the two. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also. Lead And Steel Reaction.
From www.sliderbase.com
Metals in industry Presentation Chemistry Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. — the potential difference between the metals is the driving force behind the corrosive reaction. This chart is designed to assist in broadly assessing the risk of. below is a galvanic reaction chart for dissimilar metals. there. Lead And Steel Reaction.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Lead And Steel Reaction galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each. This chart is designed to assist in broadly assessing the risk of. The anodic metal will preferentially corrode. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is. Lead And Steel Reaction.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts Lead And Steel Reaction This chart is designed to assist in broadly assessing the risk of. there are several factors affecting the rate of galvanic corrosion: — the potential difference between the metals is the driving force behind the corrosive reaction. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but. Lead And Steel Reaction.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Lead And Steel Reaction there are several factors affecting the rate of galvanic corrosion: (1) the degree of potential difference between the two. This chart is designed to assist in broadly assessing the risk of. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. The anodic. Lead And Steel Reaction.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. below is a galvanic reaction chart for dissimilar metals. The anodic metal will preferentially corrode. there are several factors affecting the rate of galvanic corrosion: This chart is designed to assist in. Lead And Steel Reaction.
From www.bbc.co.uk
What is an acid and metal reaction? BBC Bitesize Lead And Steel Reaction — the potential difference between the metals is the driving force behind the corrosive reaction. — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact. Lead And Steel Reaction.
From www.slideshare.net
Summary of Metal Reactions Lead And Steel Reaction — galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety. — the potential difference between the metals is the driving force behind the corrosive reaction. galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact. Lead And Steel Reaction.