Solid Liquid Colloid . The colloid particles are solids or liquids that are suspended in the medium. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. A gel is a colloid of solid particles in a liquid medium. In smoke, for examples, solid particles from combustion are suspended in a gas. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. These particles are larger than molecules, distinguishing a colloid from a solution. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. However, the particles in a colloid are smaller than those found in a suspension. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,.
from www.numerade.com
However, the particles in a colloid are smaller than those found in a suspension. These particles are larger than molecules, distinguishing a colloid from a solution. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. In smoke, for examples, solid particles from combustion are suspended in a gas. A gel is a colloid of solid particles in a liquid medium. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of.
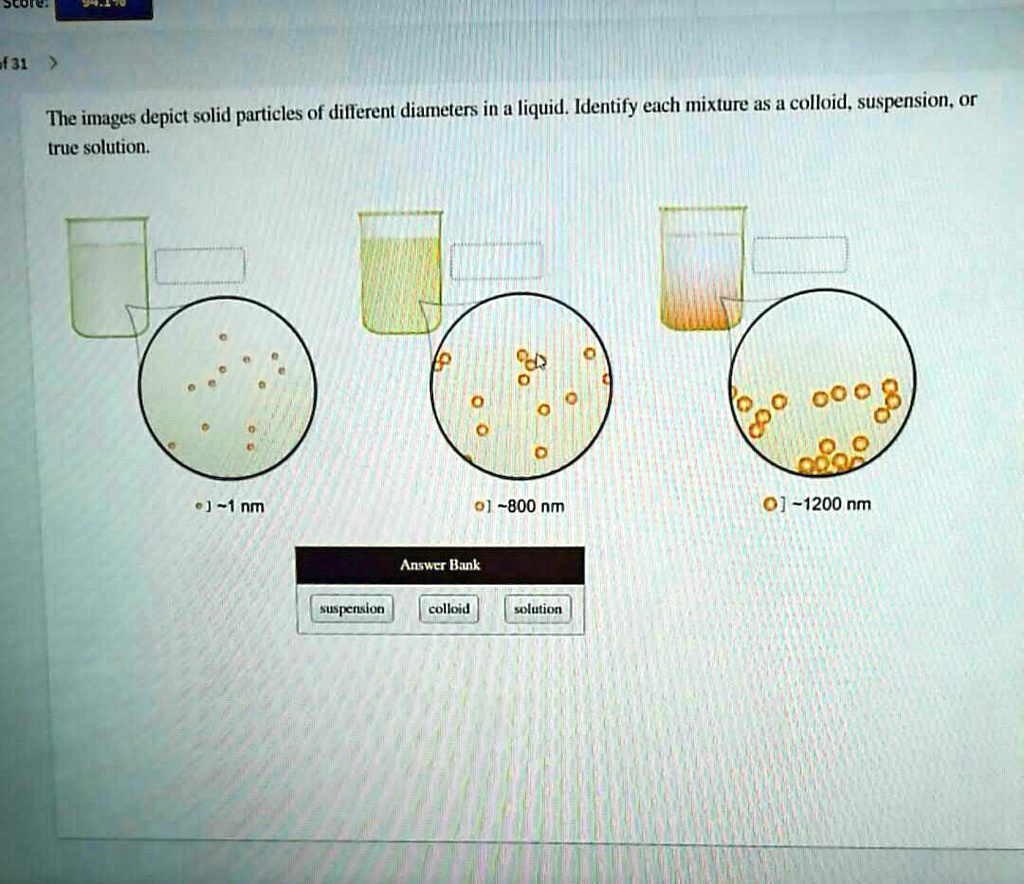
SOLVED The images depict solid particles of different diameters in a
Solid Liquid Colloid However, the particles in a colloid are smaller than those found in a suspension. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. However, the particles in a colloid are smaller than those found in a suspension. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. In smoke, for examples, solid particles from combustion are suspended in a gas. The colloid particles are solids or liquids that are suspended in the medium. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. For the various dispersion types (emulsion, gel, sol, foam, etc.),. A gel is a colloid of solid particles in a liquid medium. These particles are larger than molecules, distinguishing a colloid from a solution.
From blogs.ncl.ac.uk
kitchen chemistry STEM Newcastle Solid Liquid Colloid Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Colloids may. Solid Liquid Colloid.
From foodsciencehm.blogspot.com
Food Science Notes for 2nd Semester HM students UNIT 8 COLLOIDS Solid Liquid Colloid However, the particles in a colloid are smaller than those found in a suspension. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples. Solid Liquid Colloid.
From chem.libretexts.org
Colloids Chemistry LibreTexts Solid Liquid Colloid Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. The colloid particles are solids or liquids that are suspended in the medium. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid,. Solid Liquid Colloid.
From slideplayer.com
Types of Mixtures. ppt download Solid Liquid Colloid However, the particles in a colloid are smaller than those found in a suspension. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium.. Solid Liquid Colloid.
From www.slideserve.com
PPT Introduction to colloid and Surface chemistry PowerPoint Solid Liquid Colloid For the various dispersion types (emulsion, gel, sol, foam, etc.),. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. In smoke, for examples, solid particles from combustion are suspended in a gas. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of. Solid Liquid Colloid.
From notes.edureify.com
Colloidal chemistryintroduction to colloids EdureifyBlog Solid Liquid Colloid For the various dispersion types (emulsion, gel, sol, foam, etc.),. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. The colloid particles are solids or liquids that are suspended in the medium. Colloids may involve virtually. Solid Liquid Colloid.
From www.numerade.com
SOLVED The images depict solid particles of different diameters in a Solid Liquid Colloid These particles are larger than molecules, distinguishing a colloid from a solution. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. A gel. Solid Liquid Colloid.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Solid Liquid Colloid Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. The colloid particles are solids or liquids that are suspended in the medium. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Solid Liquid Colloid.
From www.animalia-life.club
Examples Of Colloids Mixtures Solid Liquid Colloid A gel is a colloid of solid particles in a liquid medium. However, the particles in a colloid are smaller than those found in a suspension. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples. Solid Liquid Colloid.
From uen.pressbooks.pub
Colloids Introductory Chemistry Solid Liquid Colloid A gel is a colloid of solid particles in a liquid medium. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in. Solid Liquid Colloid.
From www.flexiprep.com
Colloids Properties of True Solution, Colloidal Solution and Solid Liquid Colloid The colloid particles are solids or liquids that are suspended in the medium. For the various dispersion types (emulsion, gel, sol, foam, etc.),. A gel is a colloid of solid particles in a liquid medium. However, the particles in a colloid are smaller than those found in a suspension. Colloids are mixtures in which one or more substances are dispersed. Solid Liquid Colloid.
From basics-of-chemistry-website.blogspot.com
Basics of chemistry What is Colloidal chemistry, Colloids Solid Liquid Colloid Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. In smoke, for examples, solid particles from combustion are suspended in a gas. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Summarize the principal distinguishing. Solid Liquid Colloid.
From sciencenotes.org
What Is a Colloid? Definition and Examples Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and. Solid Liquid Colloid.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. Colloids may. Solid Liquid Colloid.
From pdfslide.net
COLLOIDS. Dispersed Systems Dispersed systems consist of particulate Solid Liquid Colloid Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. These particles are larger than molecules, distinguishing a colloid from a solution. In smoke, for examples, solid particles from combustion are suspended in a gas. Colloids may involve. Solid Liquid Colloid.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Solid Liquid Colloid Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. The colloid particles are solids or liquids that are suspended in the medium. Colloids are mixtures in which. Solid Liquid Colloid.
From byjus.com
Classification of Colloids Dispersed Phase & Dispersion Medium Solid Liquid Colloid The colloid particles are solids or liquids that are suspended in the medium. For the various dispersion types (emulsion, gel, sol, foam, etc.),. These particles are larger than molecules, distinguishing a colloid from a solution. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of.. Solid Liquid Colloid.
From www.chegg.com
Solved The following animations depict the movement of solid Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. These particles are larger than molecules, distinguishing a colloid from a solution. For the various dispersion types (emulsion, gel, sol, foam, etc.),. A gel is a colloid of solid particles in a liquid medium. The. Solid Liquid Colloid.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference Solid Liquid Colloid Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Colloids are mixtures in which one or more substances are dispersed as relatively large. Solid Liquid Colloid.
From www.slideserve.com
PPT Colloids PowerPoint Presentation ID7036943 Solid Liquid Colloid In smoke, for examples, solid particles from combustion are suspended in a gas. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. The colloid particles are solids or liquids that are suspended in the medium. These particles are larger than molecules, distinguishing a colloid from a solution. However, the particles in a colloid are smaller than those found. Solid Liquid Colloid.
From www.slideserve.com
PPT Colloidal Systems in Food Products PowerPoint Presentation ID Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. For the various dispersion types (emulsion, gel,. Solid Liquid Colloid.
From www.slideserve.com
PPT Liquid Mixtures PowerPoint Presentation, free download ID2805876 Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are. Solid Liquid Colloid.
From slideplayer.com
Suspensions and Colloids ppt download Solid Liquid Colloid Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. The colloid particles are solids or. Solid Liquid Colloid.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Colloid These particles are larger than molecules, distinguishing a colloid from a solution. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. In smoke, for examples, solid particles from combustion are suspended in a gas. Colloids are. Solid Liquid Colloid.
From kunduz.com
Homogeneous Mixtures Definition, Properties, Types, Examples Solid Liquid Colloid The colloid particles are solids or liquids that are suspended in the medium. However, the particles in a colloid are smaller than those found in a suspension. Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,.. Solid Liquid Colloid.
From en.ppt-online.org
Colloid chemistry online presentation Solid Liquid Colloid Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. These particles are larger than molecules, distinguishing a colloid from a solution. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. For the various dispersion types (emulsion, gel, sol, foam, etc.),. However, the particles in a colloid. Solid Liquid Colloid.
From www.logiota.com
Preparation and Properties of Colloids Surface Chemistry Physical Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. The colloid particles are solids or liquids that are suspended in the medium. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by. Solid Liquid Colloid.
From www.expii.com
Colloids — Definition & Examples Expii Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. A gel is a colloid of solid. Solid Liquid Colloid.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Solid Liquid Colloid However, the particles in a colloid are smaller than those found in a suspension. In smoke, for examples, solid particles from combustion are suspended in a gas. A gel is a colloid of solid particles in a liquid medium. These particles are larger than molecules, distinguishing a colloid from a solution. The colloid particles are solids or liquids that are. Solid Liquid Colloid.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 13 Colloid Chemistry PowerPoint Solid Liquid Colloid Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. However, the particles in a colloid are smaller than those found in a. Solid Liquid Colloid.
From www.vrogue.co
What Is A Colloid Definition And Examples vrogue.co Solid Liquid Colloid Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. In smoke, for examples, solid particles from combustion are suspended in a gas. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal. Solid Liquid Colloid.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. In smoke, for examples, solid particles from combustion are suspended in a gas. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,.. Solid Liquid Colloid.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Solid Liquid Colloid Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of colloidal systems given in table 4. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.), as illustrated by the examples of. For the various dispersion. Solid Liquid Colloid.
From slideplayer.com
Unit 2 Matter and Energy. ppt download Solid Liquid Colloid Familiar examples of colloids include mayonnaise, milk, fog, smoke, and gelatin. A gel is a colloid of solid particles in a liquid medium. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. The colloid particles are solids or liquids that are suspended in the medium. Summarize the. Solid Liquid Colloid.
From www.pinterest.com
Colloid Easy Science Physics concepts, Organic chemistry study Solid Liquid Colloid Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. These particles are larger than molecules, distinguishing a colloid from a solution. Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid,. Colloids are mixtures in which one or more substances are dispersed as relatively large solid. Solid Liquid Colloid.