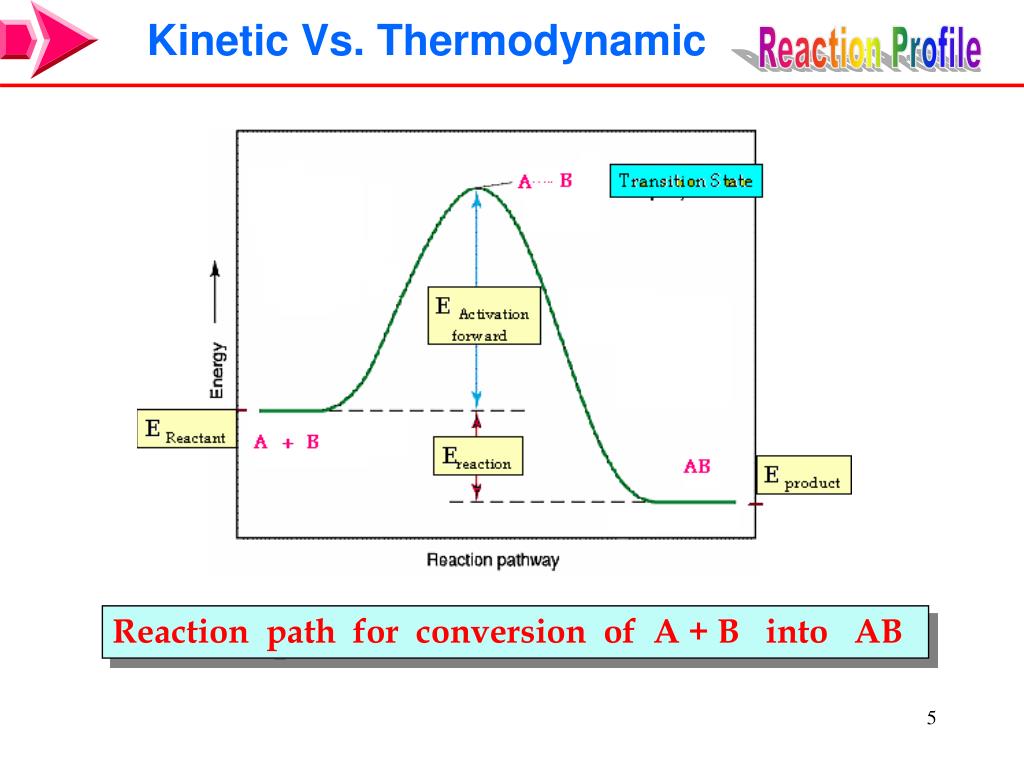
Kinetics Of Catalyst . A catalyst is a substrate that speeds up a reaction without being consumed. Catalysis is a kinetic phenomenon. Catalysts lower the activation energy barrier for a reaction without. In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. We also hear from chemist jingnan lu about. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts participate in a chemical reaction and increase its rate. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. This chapter contains sections titled: Reaction rates, reaction orders, rate.
from www.slideserve.com
Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. This chapter contains sections titled: They do not appear in the reaction’s net equation and are not consumed during the reaction. Reaction rates, reaction orders, rate. We also hear from chemist jingnan lu about. Catalysts lower the activation energy barrier for a reaction without. Catalysts participate in a chemical reaction and increase its rate. Catalysis is a kinetic phenomenon. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes.
PPT Mechanisms of Catalytic Reactions and Characterization of
Kinetics Of Catalyst Catalysis is a kinetic phenomenon. Catalysts participate in a chemical reaction and increase its rate. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. We also hear from chemist jingnan lu about. In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Reaction rates, reaction orders, rate. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. They do not appear in the reaction’s net equation and are not consumed during the reaction. This chapter contains sections titled: Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts lower the activation energy barrier for a reaction without. Catalysis is a kinetic phenomenon. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.
From www.pinterest.ph
What Is a Catalyst? Understand Catalysis Chemical Reaction Kinetics Of Catalyst We also hear from chemist jingnan lu about. In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Reaction rates, reaction orders, rate. Catalysts lower the activation energy barrier for a reaction without. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. This chapter contains sections. Kinetics Of Catalyst.
From loevndwvy.blob.core.windows.net
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog Kinetics Of Catalyst Catalysts lower the activation energy barrier for a reaction without. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Is unique in describing. Kinetics Of Catalyst.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Kinetics Of Catalyst Catalysis is a kinetic phenomenon. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. Reaction rates, reaction orders, rate. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes.. Kinetics Of Catalyst.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Kinetics Of Catalyst Reaction rates, reaction orders, rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. Catalysis is a kinetic phenomenon. A catalyst is a substrate that speeds up a reaction without being consumed. In biological systems, enzymes act. Kinetics Of Catalyst.
From 2012books.lardbucket.org
Catalysis Kinetics Of Catalyst A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Reaction rates, reaction orders, rate. We also hear from chemist jingnan lu about. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced. Kinetics Of Catalyst.
From www.slideserve.com
PPT Enzyme I PowerPoint Presentation, free download ID298555 Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysis is a kinetic phenomenon. Reaction rates, reaction orders, rate. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. A catalyst is a. Kinetics Of Catalyst.
From www.semanticscholar.org
[PDF] Concepts of modern catalysis and Semantic Scholar Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Catalysis is a kinetic phenomenon. Catalyst kinetics refers to the study of the rate at which catalytic reactions. Kinetics Of Catalyst.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Kinetics Of Catalyst A catalyst is a substrate that speeds up a reaction without being consumed. They do not appear in the reaction’s net equation and are not consumed during the reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts allow a reaction to proceed via a. Kinetics Of Catalyst.
From www.mdpi.com
Entropy Free FullText ThreeFactor Equation of Catalyst Kinetics Of Catalyst Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Reaction rates, reaction orders, rate. We also hear from chemist jingnan lu about. Catalysts lower the activation energy barrier for a reaction without. This chapter contains sections titled: Catalysts allow a reaction to proceed via a pathway. Kinetics Of Catalyst.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Kinetics Of Catalyst This chapter contains sections titled: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. We also hear from chemist jingnan lu about. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substrate that speeds up a reaction without being. Kinetics Of Catalyst.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 Kinetics Of Catalyst In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. A catalyst is a substrate that speeds up a reaction without being consumed. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts allow a reaction to proceed via a pathway that has. Kinetics Of Catalyst.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Kinetics Of Catalyst We also hear from chemist jingnan lu about. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Reaction rates, reaction orders, rate. This chapter contains sections titled:. Kinetics Of Catalyst.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a. Kinetics Of Catalyst.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Kinetics Of Catalyst This chapter contains sections titled: Catalysts participate in a chemical reaction and increase its rate. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Reaction rates, reaction orders, rate. Because a catalyst decreases the height of. Kinetics Of Catalyst.
From www.researchgate.net
Catalysis reaction Reaction of Cu(i)Lcatalysed Kinetics Of Catalyst Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Reaction rates, reaction orders, rate. Catalysis is a kinetic phenomenon. This chapter contains sections titled: Catalysts lower the activation energy barrier for a reaction without. Catalysts allow a reaction to proceed via a pathway that has a. Kinetics Of Catalyst.
From www.cell.com
Elucidation of site structures and CO oxidation of the Ir1 Kinetics Of Catalyst Catalysis is a kinetic phenomenon. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Reaction rates, reaction orders, rate. They do not appear in the reaction’s net. Kinetics Of Catalyst.
From www.researchgate.net
Hydrogenation of different catalysts. (A) Different loading Kinetics Of Catalyst Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. Catalysts participate in a chemical reaction and increase its rate. In. Kinetics Of Catalyst.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Kinetics Of Catalyst We also hear from chemist jingnan lu about. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is a kinetic phenomenon. Reaction rates, reaction orders, rate. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse.. Kinetics Of Catalyst.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Kinetics Of Catalyst They do not appear in the reaction’s net equation and are not consumed during the reaction. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst kinetics refers to the study of the rate at which catalytic. Kinetics Of Catalyst.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. This chapter contains sections titled: We also hear from chemist jingnan lu about. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysis is a kinetic phenomenon. In this lecture,. Kinetics Of Catalyst.
From www.slideserve.com
PPT Catalytic Reaction PowerPoint Presentation, free Kinetics Of Catalyst We also hear from chemist jingnan lu about. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This chapter contains sections titled: Catalysts lower the activation energy. Kinetics Of Catalyst.
From studylib.net
of catalyst deactivation by coke formation Kinetics Of Catalyst In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. This chapter contains sections titled: In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to.. Kinetics Of Catalyst.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Reaction rates, reaction orders, rate. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Catalysts participate in a chemical reaction and increase its. Kinetics Of Catalyst.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID4784681 Kinetics Of Catalyst This chapter contains sections titled: Reaction rates, reaction orders, rate. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Catalysis is a kinetic phenomenon. A catalyst is a substrate that speeds up a reaction without being consumed. They do not appear in the reaction’s net equation. Kinetics Of Catalyst.
From www.cheric.org
Chemical Reaction (Reaction rate) Kinetics Of Catalyst In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. We also hear from chemist jingnan lu about. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Catalysts participate in a chemical reaction and increase its rate.. Kinetics Of Catalyst.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Kinetics Of Catalyst This chapter contains sections titled: Catalysts participate in a chemical reaction and increase its rate. We also hear from chemist jingnan lu about. Catalysis is a kinetic phenomenon. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Reaction rates, reaction orders, rate. They do not appear in the reaction’s net. Kinetics Of Catalyst.
From rumble.com
Catalysis, Chemistry Kinetics Of Catalyst Catalysis is a kinetic phenomenon. Reaction rates, reaction orders, rate. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Catalysts participate in a chemical reaction and increase its rate. This chapter contains sections titled: Catalysts lower. Kinetics Of Catalyst.
From www.youtube.com
11 Catalysts YouTube Kinetics Of Catalyst We also hear from chemist jingnan lu about. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Reaction rates, reaction orders, rate. Is unique in describing how to conduct kinetic. Kinetics Of Catalyst.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts lower the activation energy barrier for a reaction without. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substrate that speeds up a reaction without being consumed. In biological systems, enzymes. Kinetics Of Catalyst.
From www.researchgate.net
Reaction as a function of catalyst Download Scientific Diagram Kinetics Of Catalyst In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Catalysts lower the activation energy barrier for a reaction without. This chapter contains sections titled: A catalyst is a substrate that speeds up a reaction without being consumed. We also hear from chemist jingnan lu about. Catalysis is a kinetic phenomenon.. Kinetics Of Catalyst.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Kinetics Of Catalyst Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. A catalyst is a substrate that speeds up. Kinetics Of Catalyst.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Kinetics Of Catalyst In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from \(10^3\) to. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. In this lecture, catalysts of different types are introduced, including nature’s catalysts, enzymes. Catalysts allow a reaction to proceed via a pathway that has a lower activation. Kinetics Of Catalyst.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Kinetics Of Catalyst Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Reaction rates, reaction orders, rate. In this lecture, catalysts of different types are introduced,. Kinetics Of Catalyst.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Kinetics Of Catalyst Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and. Catalysts participate in a chemical reaction and increase its rate. In biological systems, enzymes. Kinetics Of Catalyst.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Kinetics Of Catalyst A catalyst is a substrate that speeds up a reaction without being consumed. Is unique in describing how to conduct kinetic experiments with heterogeneous catalysts, analyze and. This chapter contains sections titled: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst kinetics refers to the study of the rate. Kinetics Of Catalyst.