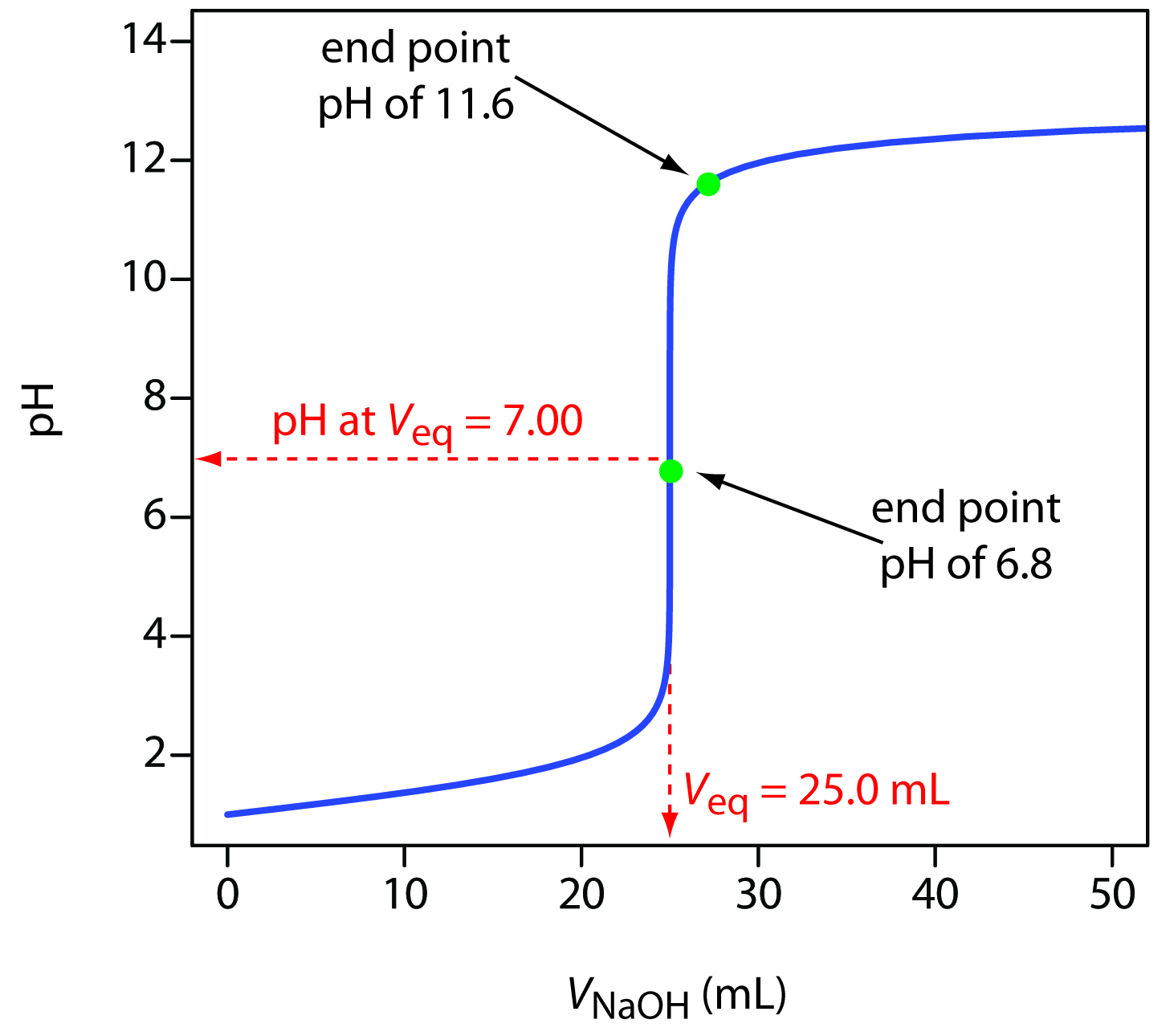
Titration Curve Chemistry . The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The equivalence point of a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. It is based on the neutralization reaction, where. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
from chem.libretexts.org
A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. The end point of a titration is the point at which an indicator changes. It is based on the neutralization reaction, where. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The figure below shows two different examples of a strong. The titration curve is a graphical representation of the ph or other property changes during a titration experiment.
9.1 Overview of Titrimetry Chemistry LibreTexts
Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. It is based on the neutralization reaction, where. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. It provides valuable information about the reaction under study and helps. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Chemistry It is based on the neutralization reaction, where. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as. Titration Curve Chemistry.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Chemistry The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It is based on the neutralization reaction, where. The titration curve. Titration Curve Chemistry.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Chemistry Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The equivalence point of a titration. A. Titration Curve Chemistry.
From fyofhmvuw.blob.core.windows.net
Titration Curve Meaning at Julia Cable blog Titration Curve Chemistry The end point of a titration is the point at which an indicator changes. It is based on the neutralization reaction, where. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic. Titration Curve Chemistry.
From www.savemyexams.com
Titration Curves of Polyprotic Acids College Board AP® Chemistry Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which. Titration Curve Chemistry.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Chemistry It is based on the neutralization reaction, where. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below shows. Titration Curve Chemistry.
From fyofhmvuw.blob.core.windows.net
Titration Curve Meaning at Julia Cable blog Titration Curve Chemistry It provides valuable information about the reaction under study and helps. The equivalence point of a titration. The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different. Titration Curve Chemistry.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Curve Chemistry It provides valuable information about the reaction under study and helps. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. Titration Curve Chemistry.
From giocuucqp.blob.core.windows.net
Titration Exam Questions A Level Chemistry Aqa at Angela Gardner blog Titration Curve Chemistry Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. It provides valuable information about the reaction under study and helps. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below shows two different examples of a strong. A. Titration Curve Chemistry.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Titration Curve Chemistry It is based on the neutralization reaction, where. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. The equivalence point of a. Titration Curve Chemistry.
From mungfali.com
Buffer Region On Titration Curve Titration Curve Chemistry The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. It provides valuable information about the reaction under study and helps. It is based on the neutralization reaction,. Titration Curve Chemistry.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Chemistry The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph. Titration Curve Chemistry.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often. Titration Curve Chemistry.
From gioeaehpw.blob.core.windows.net
Indicators Chemistry A Level at Johnnie Andrews blog Titration Curve Chemistry The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration.. Titration Curve Chemistry.
From fyofhmvuw.blob.core.windows.net
Titration Curve Meaning at Julia Cable blog Titration Curve Chemistry The figure below shows two different examples of a strong. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. The end point of a titration is the point at which an indicator changes. Titrations are often. Titration Curve Chemistry.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Chemistry Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of. Titration Curve Chemistry.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Chemistry Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong.. Titration Curve Chemistry.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Curve Chemistry The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical. Titration Curve Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The equivalence point of a titration. It is based on the neutralization reaction, where. Titration curves show how the ph of an acidic or. Titration Curve Chemistry.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Chemistry The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The end point of a titration is the point at which an indicator changes. The equivalence point of a titration. It is based on the neutralization reaction, where. Titration curves show how the ph of an acidic or basic solution changes as. Titration Curve Chemistry.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The figure below shows two different examples of a strong. The titration curve is a graphical representation of the ph or other property changes. Titration Curve Chemistry.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Chemistry The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It is based on the neutralization reaction,. Titration Curve Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Chemistry It provides valuable information about the reaction under study and helps. The figure below shows two different examples of a strong. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It is based on the neutralization reaction, where. A titration curve is a graphical representation of the ph of a solution. Titration Curve Chemistry.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Chemistry It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic. Titration Curve Chemistry.
From giocuucqp.blob.core.windows.net
Titration Exam Questions A Level Chemistry Aqa at Angela Gardner blog Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration. Titration Curve Chemistry.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic. Titration Curve Chemistry.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Chemistry It is based on the neutralization reaction, where. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain. Titration Curve Chemistry.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. The equivalence point of a titration. The titration curve is a graphical representation of. Titration Curve Chemistry.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Chemistry It provides valuable information about the reaction under study and helps. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. The titration curve is a graphical representation of the ph or other property changes during. Titration Curve Chemistry.
From philschatz.com
AcidBase Titrations · Chemistry Titration Curve Chemistry The figure below shows two different examples of a strong. It provides valuable information about the reaction under study and helps. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the. Titration Curve Chemistry.
From mungfali.com
Titration Curve Graph Titration Curve Chemistry The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The equivalence point. Titration Curve Chemistry.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Chemistry Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under. Titration Curve Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Chemistry The end point of a titration is the point at which an indicator changes. It is based on the neutralization reaction, where. It provides valuable information about the reaction under study and helps. The equivalence point of a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. A. Titration Curve Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curve Chemistry The equivalence point of a titration. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The. Titration Curve Chemistry.
From generalchemistrylab.blogspot.co.uk
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Chemistry Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. The. Titration Curve Chemistry.