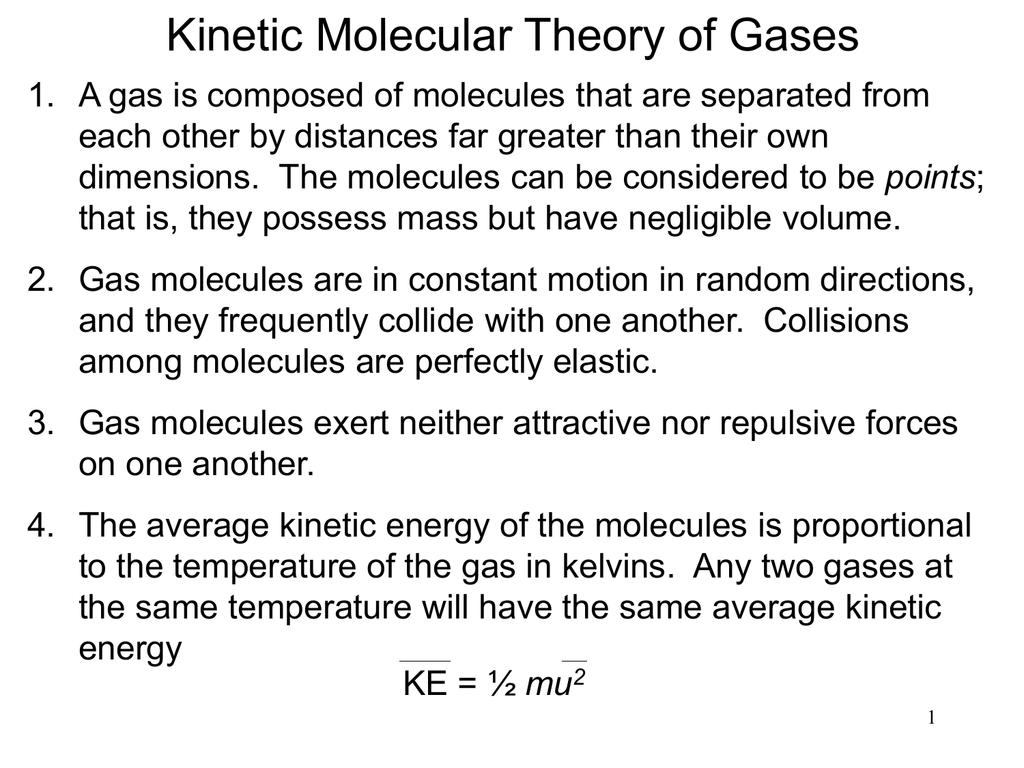
What Are The Five Points Of The Kinetic Molecular Theory . This theory is based on the following five postulates described here. This theory is based on the following five postulates described here. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). The term “molecule” will be used to refer to the individual. The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. Kinetic molecular theory states that gas particles are in constant. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. A gas is composed of molecules that are separated by average.
from studylib.net
The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). A gas is composed of molecules that are separated by average. Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. The term “molecule” will be used to refer to the individual. Kinetic molecular theory states that gas particles are in constant. This theory is based on the following five postulates described here. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical.
Molecular Theory Notes
What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. This theory helps explain observable properties and behaviors of solids, liquids, and gases. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. Kinetic molecular theory states that gas particles are in constant. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). This theory is based on the following five postulates described here. A gas is composed of molecules that are separated by average.
From www.slideserve.com
PPT The Molecular Theory of Matter and Motion PowerPoint What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. This theory is based on the following five postulates described here. Kinetic molecular theory states that gas particles are in constant. Express the five basic assumptions of the kinetic molecular theory of gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship between. What Are The Five Points Of The Kinetic Molecular Theory.
From sciencenotes.org
Molecular Theory of Gases What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. A gas is composed of molecules that are separated by average. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. The particle theory of matter or. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT KMT PowerPoint Presentation, free download ID6901291 What Are The Five Points Of The Kinetic Molecular Theory Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The term “molecule” will be used to refer to the individual. The particle theory of matter or the kinetic molecular theory of matter describes. What Are The Five Points Of The Kinetic Molecular Theory.
From es.slideshare.net
Molecular Theory What Are The Five Points Of The Kinetic Molecular Theory Express the five basic assumptions of the kinetic molecular theory of gases. The term “molecule” will be used to refer to the individual. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. Kinetic molecular theory states that gas particles are in constant. The particle theory of matter or. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. Express the five basic assumptions of the kinetic molecular theory of gases. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. The term “molecule”. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory PowerPoint Presentation, free download What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. A gas is composed of molecules that are separated by average. This theory is based on the following five postulates described here. This theory is based on the following five postulates described here. This theory helps explain. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download What Are The Five Points Of The Kinetic Molecular Theory This theory helps explain observable properties and behaviors of solids, liquids, and gases. A gas is composed of molecules that are separated by average. This theory is based on the following five postulates described here. Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. The particle. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint What Are The Five Points Of The Kinetic Molecular Theory Kinetic molecular theory states that gas particles are in constant. Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The term “molecule” will be used to refer to the individual. The postulates of. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. Kinetic molecular theory states that gas particles are in constant. This theory is based on the following five postulates described here. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. Express the five basic assumptions of the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT The Theory of Matter PowerPoint Presentation What Are The Five Points Of The Kinetic Molecular Theory The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). Kinetic molecular theory states that gas particles are in constant. This theory is based on the following five postulates described here. A gas is composed. What Are The Five Points Of The Kinetic Molecular Theory.
From studylib.net
The Molecular Theory of Liquids & Solids What Are The Five Points Of The Kinetic Molecular Theory Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. The term “molecule” will be used to refer to the individual. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic. What Are The Five Points Of The Kinetic Molecular Theory.
From selfeducateor.blogspot.com
Study Time Molecular Theory (KTG) (introduction and Explanation) What Are The Five Points Of The Kinetic Molecular Theory This theory helps explain observable properties and behaviors of solids, liquids, and gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. What Are The Five Points Of The Kinetic Molecular Theory.
From www.sliderbase.com
Molecular Theory Presentation Chemistry What Are The Five Points Of The Kinetic Molecular Theory This theory helps explain observable properties and behaviors of solids, liquids, and gases. A gas is composed of molecules that are separated by average. The term “molecule” will be used to refer to the individual. This theory is based on the following five postulates described here. The particle theory of matter or the kinetic molecular theory of matter describes the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. This theory helps explain observable properties and behaviors of solids, liquids, and gases. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. The particle theory of matter or the kinetic molecular theory of matter describes the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory PowerPoint Presentation, free download What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. The term “molecule” will be used to refer to the individual. Kinetic molecular theory states that gas particles are in constant. A gas is composed of. What Are The Five Points Of The Kinetic Molecular Theory.
From karsonsrrichards.blogspot.com
Molecular Theory of Gases KarsonsrRichards What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). The postulates of the kinetic molecular theory provide us a way to understand the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory (KMT) PowerPoint Presentation, free What Are The Five Points Of The Kinetic Molecular Theory Kinetic molecular theory states that gas particles are in constant. Express the five basic assumptions of the kinetic molecular theory of gases. The term “molecule” will be used to refer to the individual. This theory helps explain observable properties and behaviors of solids, liquids, and gases. This theory is based on the following five postulates described here. This theory is. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideshare.net
Molecular Theory What Are The Five Points Of The Kinetic Molecular Theory The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). A gas is composed of molecules that are separated by average. Express the five basic assumptions of the kinetic molecular theory of gases. This theory. What Are The Five Points Of The Kinetic Molecular Theory.
From rumble.com
molecular theory, ideal gases Chemistry What Are The Five Points Of The Kinetic Molecular Theory A gas is composed of molecules that are separated by average. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). This theory is based on the following five postulates described here. The term “molecule”. What Are The Five Points Of The Kinetic Molecular Theory.
From studylib.net
Molecular Theory Notes What Are The Five Points Of The Kinetic Molecular Theory The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. Kinetic molecular theory states. What Are The Five Points Of The Kinetic Molecular Theory.
From dbzildzjeco.blob.core.windows.net
What Is Molecular Theory In Physics at Marie Upton blog What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. The term “molecule” will be used to refer to the individual. This theory helps explain observable properties and behaviors of solids, liquids, and gases. A gas is composed of molecules that are separated by average. The postulates. What Are The Five Points Of The Kinetic Molecular Theory.
From studylib.net
Molecular Theory What Are The Five Points Of The Kinetic Molecular Theory Express the five basic assumptions of the kinetic molecular theory of gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. The term “molecule” will be used to refer to the individual. This theory is based on the following five postulates described here. A gas is composed of. What Are The Five Points Of The Kinetic Molecular Theory.
From www.youtube.com
Chemistry 7.3 Molecular Theory YouTube What Are The Five Points Of The Kinetic Molecular Theory Express the five basic assumptions of the kinetic molecular theory of gases. A gas is composed of molecules that are separated by average. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). This theory. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT 5.7/5.1 Molecular Theory of Gases PowerPoint Presentation What Are The Five Points Of The Kinetic Molecular Theory A gas is composed of molecules that are separated by average. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. The postulates of the kinetic molecular theory provide us a way to understand the relationship. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Section 10.5 The Molecular Theory PowerPoint Presentation What Are The Five Points Of The Kinetic Molecular Theory Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. This theory is based on the following five postulates described here. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). This theory is. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT CHAPTER 12 GASES AND THEORY PowerPoint What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. This theory is based on the following five postulates described here. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). Express the five. What Are The Five Points Of The Kinetic Molecular Theory.
From www.youtube.com
1 Molecular Theory YouTube What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The term “molecule” will be used to refer to the individual. The term “molecule” will be used to refer to the individual. The particle theory of matter or the kinetic molecular theory of matter describes the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory PowerPoint Presentation, free download What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. The term “molecule” will be used to refer to the individual. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The particle theory of matter or the kinetic molecular theory of matter describes the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.chem.uwec.edu
molecular theory of matter What Are The Five Points Of The Kinetic Molecular Theory The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). The term “molecule” will be used to refer to the individual. A gas is composed of molecules that are separated by average. Kinetic molecular theory. What Are The Five Points Of The Kinetic Molecular Theory.
From www.youtube.com
Molecular Theory YouTube What Are The Five Points Of The Kinetic Molecular Theory The postulates of the kinetic molecular theory provide us a way to understand the relationship between molecular properties and the physical. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. Kinetic molecular theory states that gas particles are in constant. Express the five basic assumptions of the. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Are The Five Points Of The Kinetic Molecular Theory A gas is composed of molecules that are separated by average. Kinetic molecular theory states that gas particles are in constant. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of. What Are The Five Points Of The Kinetic Molecular Theory.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Are The Five Points Of The Kinetic Molecular Theory Kinetic molecular theory states that gas particles are in constant. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. This theory helps explain observable properties and behaviors of solids, liquids, and gases. A gas is composed of molecules that are separated by average. This theory is based. What Are The Five Points Of The Kinetic Molecular Theory.
From lessonlibraryoptimate.z14.web.core.windows.net
Molecular Theory Explained What Are The Five Points Of The Kinetic Molecular Theory This theory is based on the following five postulates described here. This theory helps explain observable properties and behaviors of solids, liquids, and gases. This theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual. Kinetic molecular theory states that gas particles are in constant. Express the five basic. What Are The Five Points Of The Kinetic Molecular Theory.
From slideplayer.com
Molecular Theory and Gases ppt download What Are The Five Points Of The Kinetic Molecular Theory The term “molecule” will be used to refer to the individual. Express the five basic assumptions of the kinetic molecular theory of gases. This theory is based on the following five postulates described here. A gas is composed of molecules that are separated by average. This theory is based on the following five postulates described here. Kinetic molecular theory states. What Are The Five Points Of The Kinetic Molecular Theory.