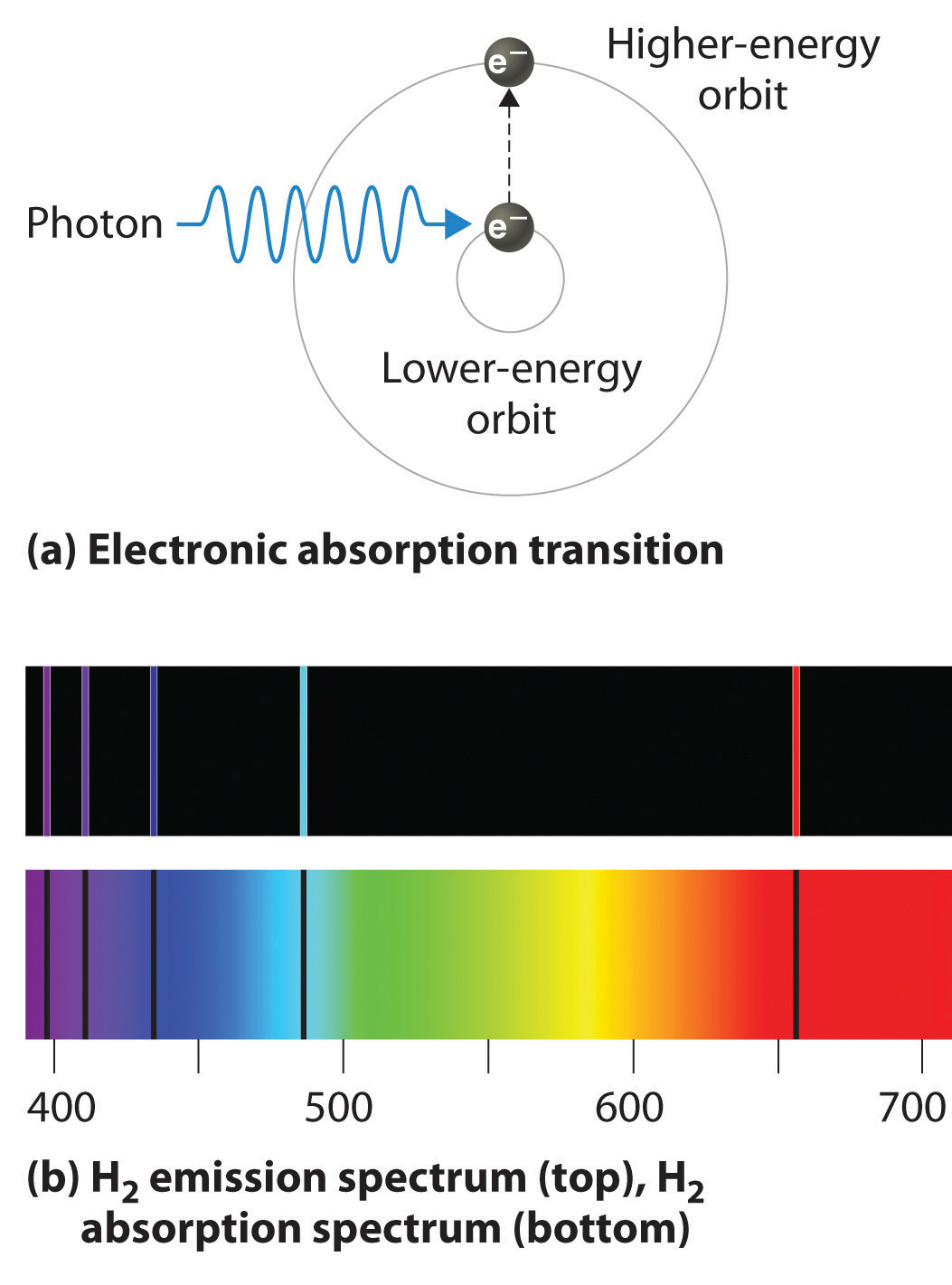
What Happens When Electron Absorbs Photon . The electrons will absorb the energy of the light wave and change their energy state. There are several options that could happen next, either. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; Electrons don’t like being in an excited state, and so fall back. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. Once the electron absorbs a photon, it is excited by the energy. After the electron is excited, it drops down and releases a photon with the energy difference between the two. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. The energy from the photon partially goes over to the electron and the electron moves faster. If a photon hits the electron, both moving against each other, the electron gets. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The same interaction that admits this pushing and pulling is the.
from chem.libretexts.org
There are several options that could happen next, either. Electrons don’t like being in an excited state, and so fall back. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Once the electron absorbs a photon, it is excited by the energy. If a photon hits the electron, both moving against each other, the electron gets. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The same interaction that admits this pushing and pulling is the. The electrons will absorb the energy of the light wave and change their energy state. The energy from the photon partially goes over to the electron and the electron moves faster. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus;
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts
What Happens When Electron Absorbs Photon There are several options that could happen next, either. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. There are several options that could happen next, either. If a photon hits the electron, both moving against each other, the electron gets. The energy from the photon partially goes over to the electron and the electron moves faster. Electrons don’t like being in an excited state, and so fall back. Once the electron absorbs a photon, it is excited by the energy. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The same interaction that admits this pushing and pulling is the. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. The electrons will absorb the energy of the light wave and change their energy state. After the electron is excited, it drops down and releases a photon with the energy difference between the two.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube What Happens When Electron Absorbs Photon When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. Electrons don’t like being in an excited state, and so fall back. Charges absorb and emit. What Happens When Electron Absorbs Photon.
From www.pinterest.com
Light and photosynthetic pigments The lightdependent reactions What Happens When Electron Absorbs Photon The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. Electrons don’t like being in an excited state, and so fall back. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. Charges absorb and emit photons,. What Happens When Electron Absorbs Photon.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps What Happens When Electron Absorbs Photon Charges absorb and emit photons, and that's how they experience the electromagnetic force. After the electron is excited, it drops down and releases a photon with the energy difference between the two. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. Electrons. What Happens When Electron Absorbs Photon.
From studylib.net
Process of Photosynthesis What Happens When Electron Absorbs Photon There are several options that could happen next, either. After the electron is excited, it drops down and releases a photon with the energy difference between the two. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. (a) when a hydrogen atom absorbs a photon of. What Happens When Electron Absorbs Photon.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 What Happens When Electron Absorbs Photon Charges absorb and emit photons, and that's how they experience the electromagnetic force. The same interaction that admits this pushing and pulling is the. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. The atom has energy levels, and if the photon. What Happens When Electron Absorbs Photon.
From theory.labster.com
Speed and Number of Photoelectrons in the Photon Model Labster What Happens When Electron Absorbs Photon Electrons don’t like being in an excited state, and so fall back. If a photon hits the electron, both moving against each other, the electron gets. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. Conservation of energy determines the energy of the photon and. What Happens When Electron Absorbs Photon.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology What Happens When Electron Absorbs Photon (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. There are several options that could happen next, either. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; The same interaction that. What Happens When Electron Absorbs Photon.
From chem.libretexts.org
7.1 Vocabulary Chemistry LibreTexts What Happens When Electron Absorbs Photon The same interaction that admits this pushing and pulling is the. After the electron is excited, it drops down and releases a photon with the energy difference between the two. The energy from the photon partially goes over to the electron and the electron moves faster. An atom can absorb or emit one photon when an electron makes a transition. What Happens When Electron Absorbs Photon.
From www.chegg.com
Solved What happens to an electron when it absorbs a photon What Happens When Electron Absorbs Photon When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; If a photon hits the electron, both moving against each other, the electron gets. After the electron is excited, it drops down and releases a photon with the energy difference between the two. The energy from the photon partially goes. What Happens When Electron Absorbs Photon.
From slideplayer.com
SEMESTER I EXAM REVIEW B ppt download What Happens When Electron Absorbs Photon There are several options that could happen next, either. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. Electrons don’t like being in an excited state, and. What Happens When Electron Absorbs Photon.
From quizlet.com
Explain what happens when an atom absorbs a photon. Draw a p Quizlet What Happens When Electron Absorbs Photon If a photon hits the electron, both moving against each other, the electron gets. After the electron is excited, it drops down and releases a photon with the energy difference between the two. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n.. What Happens When Electron Absorbs Photon.
From study.com
Electron Transition Definition, Chart & Examples Lesson What Happens When Electron Absorbs Photon Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. The same interaction that admits this pushing and pulling is the. Charges absorb and emit photons, and that's how they experience the electromagnetic force. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away. What Happens When Electron Absorbs Photon.
From www.numerade.com
SOLVEDWhat happens to an electron as it absorbs a photon of light? What Happens When Electron Absorbs Photon When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; After the electron is excited, it drops down and releases a photon with the energy difference between the two. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher. What Happens When Electron Absorbs Photon.
From www.jobilize.com
What happens when a compound absorbs a photon of light By OpenStax What Happens When Electron Absorbs Photon Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. Electrons don’t like being in an excited state, and so fall back. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; Charges absorb and emit photons, and that's how they. What Happens When Electron Absorbs Photon.
From www.researchgate.net
2 Schematic sketch of photoelectric effect An electron absorbs the What Happens When Electron Absorbs Photon When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; If a photon hits the electron, both moving against each other, the electron gets. The energy from the photon partially goes over to the electron and the electron moves faster. The atom has energy levels, and if the photon energy. What Happens When Electron Absorbs Photon.
From brainly.com
What occurs when an atom absorbs a photon of light energy an electron What Happens When Electron Absorbs Photon After the electron is excited, it drops down and releases a photon with the energy difference between the two. There are several options that could happen next, either. If a photon hits the electron, both moving against each other, the electron gets. The energy from the photon partially goes over to the electron and the electron moves faster. Electrons don’t. What Happens When Electron Absorbs Photon.
From www.youtube.com
ABC Zoom Electrons and photons absorption and transmission of light What Happens When Electron Absorbs Photon The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. Electrons don’t like being in an excited state, and so fall back. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The energy from the photon partially goes over to the electron. What Happens When Electron Absorbs Photon.
From www.chegg.com
Solved 11. A hydrogen atom absorbs a photon of UV light, and What Happens When Electron Absorbs Photon Once the electron absorbs a photon, it is excited by the energy. Charges absorb and emit photons, and that's how they experience the electromagnetic force. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; The energy from the photon partially goes over to the electron and the electron moves. What Happens When Electron Absorbs Photon.
From www.slideserve.com
PPT The Electron PowerPoint Presentation, free download ID2061737 What Happens When Electron Absorbs Photon Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. There are several options that could happen next, either. The same interaction that admits this pushing and pulling is the. After the electron is excited, it drops down and releases a photon with the energy difference between the two. An atom. What Happens When Electron Absorbs Photon.
From energywavetheory.com
What is Quantum? EWT What Happens When Electron Absorbs Photon When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; After the electron is excited, it drops down and releases a photon with the energy difference between the two. Electrons don’t like being in an excited state, and so fall back. The energy from the photon partially goes over to. What Happens When Electron Absorbs Photon.
From energywavetheory.com
Photon Creation and Absorption EWT What Happens When Electron Absorbs Photon Once the electron absorbs a photon, it is excited by the energy. Electrons don’t like being in an excited state, and so fall back. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. When electrons absorb energy, they become ‘excited’ and move to higher energy. What Happens When Electron Absorbs Photon.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Happens When Electron Absorbs Photon There are several options that could happen next, either. Electrons don’t like being in an excited state, and so fall back. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. After the electron is excited, it drops down and releases a photon with the energy difference between the two. The. What Happens When Electron Absorbs Photon.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint What Happens When Electron Absorbs Photon The same interaction that admits this pushing and pulling is the. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The electrons will absorb the energy of the light wave and change their energy state. There are several options that could happen next, either. The energy from the photon partially goes over to the electron and. What Happens When Electron Absorbs Photon.
From www.sciencenews.org
Colliding photons made matter. But are the photons ‘real’? Science News What Happens When Electron Absorbs Photon The same interaction that admits this pushing and pulling is the. Electrons don’t like being in an excited state, and so fall back. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; If a photon hits the electron, both moving against each other, the electron gets. Charges absorb and. What Happens When Electron Absorbs Photon.
From www.chegg.com
Solved What happens when a molecular absorbs a photon? What What Happens When Electron Absorbs Photon Charges absorb and emit photons, and that's how they experience the electromagnetic force. The electrons will absorb the energy of the light wave and change their energy state. The same interaction that admits this pushing and pulling is the. If a photon hits the electron, both moving against each other, the electron gets. Conservation of energy determines the energy of. What Happens When Electron Absorbs Photon.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 What Happens When Electron Absorbs Photon If a photon hits the electron, both moving against each other, the electron gets. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. Once the electron absorbs a photon, it is excited by the energy. (a) when a hydrogen atom absorbs a photon of light,. What Happens When Electron Absorbs Photon.
From slideplayer.com
Chapter 7 Light and Color ppt download What Happens When Electron Absorbs Photon Once the electron absorbs a photon, it is excited by the energy. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; The electrons will absorb the energy of the light wave and change their energy state. Conservation of energy determines the energy of the photon and thus the frequency. What Happens When Electron Absorbs Photon.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps What Happens When Electron Absorbs Photon Charges absorb and emit photons, and that's how they experience the electromagnetic force. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and. What Happens When Electron Absorbs Photon.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts What Happens When Electron Absorbs Photon The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. The electrons will absorb the energy of the light wave and change their energy state. After the electron is excited, it drops down and releases a photon with the energy difference between the two. Conservation of. What Happens When Electron Absorbs Photon.
From study.com
Bohr Atomic Model Overview & Examples Lesson What Happens When Electron Absorbs Photon Charges absorb and emit photons, and that's how they experience the electromagnetic force. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus; The same interaction that admits this pushing and. What Happens When Electron Absorbs Photon.
From www.numerade.com
SOLVEDWhat happens to an electron as it absorbs a photon of light? What Happens When Electron Absorbs Photon There are several options that could happen next, either. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. After the electron is excited, it drops down and releases a photon with the energy difference between the two. When electrons absorb energy, they. What Happens When Electron Absorbs Photon.
From energywavetheory.com
Photon Creation and Absorption EWT What Happens When Electron Absorbs Photon Charges absorb and emit photons, and that's how they experience the electromagnetic force. The same interaction that admits this pushing and pulling is the. Once the electron absorbs a photon, it is excited by the energy. There are several options that could happen next, either. Conservation of energy determines the energy of the photon and thus the frequency of the. What Happens When Electron Absorbs Photon.
From courses.lumenlearning.com
The LightDependent Reactions of Photosynthesis Biology I What Happens When Electron Absorbs Photon (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition. There are several options that could happen next, either.. What Happens When Electron Absorbs Photon.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy What Happens When Electron Absorbs Photon Once the electron absorbs a photon, it is excited by the energy. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. After the electron is excited, it. What Happens When Electron Absorbs Photon.
From askfilo.com
When an electron in the hydrogen atom in ground state absorbs a photon of.. What Happens When Electron Absorbs Photon Once the electron absorbs a photon, it is excited by the energy. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The same interaction that admits this pushing and pulling is the. The atom has energy levels, and if the photon energy coincides (within a small $δe$, the width of the energy level) with the transition.. What Happens When Electron Absorbs Photon.