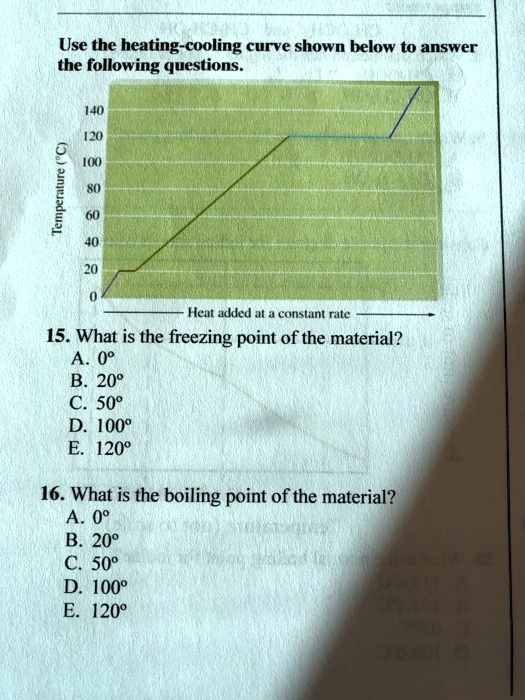
Heating Curve Questions And Answers . Answer sheet answer the following using the above heating curve 1. What is the melting temperature of the above substance? It represents the heating of substance x at a constant rate of. Base your answers to questions 40 through 42 on the information below. Starting as a gas at 206°c, a sample of a substance is allowed to cool for. Evaporation of sweat requires energy and thus take excess heat away from. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. From the phase diagram for water, determine the state of water at: If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. The heating curve shown above is a plot of temperature vs time. Practice the experiment before you. Use the heating curve to explain why the temperature did not increase during state changes.
from amiahgokeestes.blogspot.com
I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. The heating curve shown above is a plot of temperature vs time. Evaporation of sweat requires energy and thus take excess heat away from. Use the heating curve to explain why the temperature did not increase during state changes. From the phase diagram for water, determine the state of water at: Practice the experiment before you. It represents the heating of substance x at a constant rate of. Starting as a gas at 206°c, a sample of a substance is allowed to cool for. What is the melting temperature of the above substance? Base your answers to questions 40 through 42 on the information below.
Use the Heating Curve Below to Answer the Following Questions
Heating Curve Questions And Answers From the phase diagram for water, determine the state of water at: What is the melting temperature of the above substance? The heating curve shown above is a plot of temperature vs time. Starting as a gas at 206°c, a sample of a substance is allowed to cool for. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Answer sheet answer the following using the above heating curve 1. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Base your answers to questions 40 through 42 on the information below. From the phase diagram for water, determine the state of water at: It represents the heating of substance x at a constant rate of. Practice the experiment before you. Use the heating curve to explain why the temperature did not increase during state changes. Evaporation of sweat requires energy and thus take excess heat away from.
From studylib.net
Calorimetry practice Heating Curve Questions And Answers It represents the heating of substance x at a constant rate of. Use the heating curve to explain why the temperature did not increase during state changes. From the phase diagram for water, determine the state of water at: Practice the experiment before you. Base your answers to questions 40 through 42 on the information below. If a substance is. Heating Curve Questions And Answers.
From www.chegg.com
Solved Use the heating curve provided to answer the Heating Curve Questions And Answers Use the heating curve to explain why the temperature did not increase during state changes. Practice the experiment before you. Starting as a gas at 206°c, a sample of a substance is allowed to cool for. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. The heating curve. Heating Curve Questions And Answers.
From www.coursehero.com
[Solved] Worksheet 9.3 HEATING CURVES 1. a) What... Course Hero Heating Curve Questions And Answers Starting as a gas at 206°c, a sample of a substance is allowed to cool for. The heating curve shown above is a plot of temperature vs time. From the phase diagram for water, determine the state of water at: I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance.. Heating Curve Questions And Answers.
From www.chegg.com
Solved The graph shown below is the hypothetical heating Heating Curve Questions And Answers Evaporation of sweat requires energy and thus take excess heat away from. What is the melting temperature of the above substance? If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of. Heating Curve Questions And Answers.
From quizizz.com
Heating Curves questions & answers for quizzes and tests Quizizz Heating Curve Questions And Answers Evaporation of sweat requires energy and thus take excess heat away from. Answer sheet answer the following using the above heating curve 1. It represents the heating of substance x at a constant rate of. Practice the experiment before you. Use the heating curve to explain why the temperature did not increase during state changes. From the phase diagram for. Heating Curve Questions And Answers.
From studytofux1066t.z21.web.core.windows.net
Heating And Cooling Curves Worksheets Heating Curve Questions And Answers Use the heating curve to explain why the temperature did not increase during state changes. What is the melting temperature of the above substance? If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Practice the experiment before you. The heating curve shown above is a plot of temperature. Heating Curve Questions And Answers.
From learningcampusstall.z21.web.core.windows.net
Heating And Cooling Curves Worksheet Heating Curve Questions And Answers The heating curve shown above is a plot of temperature vs time. Practice the experiment before you. Answer sheet answer the following using the above heating curve 1. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. What is the melting temperature of the above substance? Evaporation of sweat. Heating Curve Questions And Answers.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Questions And Answers What is the melting temperature of the above substance? It represents the heating of substance x at a constant rate of. Evaporation of sweat requires energy and thus take excess heat away from. Base your answers to questions 40 through 42 on the information below. Answer sheet answer the following using the above heating curve 1. Use the heating curve. Heating Curve Questions And Answers.
From studytofux1066t.z21.web.core.windows.net
Heating Curve Worksheets Heating Curve Questions And Answers If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Use the heating curve to explain why the temperature did not increase during state changes. What is the melting temperature of the above substance? From the phase diagram for water, determine the state of water at: It represents the. Heating Curve Questions And Answers.
From studylib.net
IB1 Physics Heating Curve of Water Lab Heating Curve Questions And Answers I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. What is the melting temperature of the above substance? Answer sheet answer the following using the above heating curve 1. Base your answers to questions 40 through 42 on the information below. From the phase diagram for water, determine the. Heating Curve Questions And Answers.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Questions And Answers Answer sheet answer the following using the above heating curve 1. Starting as a gas at 206°c, a sample of a substance is allowed to cool for. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. It represents the heating of substance x at a constant rate of.. Heating Curve Questions And Answers.
From amiahgokeestes.blogspot.com
Use the Heating Curve Below to Answer the Following Questions Heating Curve Questions And Answers If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Practice the experiment before you. Base your answers to questions 40 through 42 on the information below. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Starting as. Heating Curve Questions And Answers.
From printablejacob.z22.web.core.windows.net
Heating And Cooling Curve Questions Heating Curve Questions And Answers Base your answers to questions 40 through 42 on the information below. Answer sheet answer the following using the above heating curve 1. Use the heating curve to explain why the temperature did not increase during state changes. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. The. Heating Curve Questions And Answers.
From www.vrogue.co
Heating And Cooling Curves Worksheet Educational Work vrogue.co Heating Curve Questions And Answers I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Use the heating curve to explain why the temperature did not increase during state changes. It represents the heating. Heating Curve Questions And Answers.
From davida.davivienda.com
Heating Curve Worksheet 2 Printable Word Searches Heating Curve Questions And Answers Base your answers to questions 40 through 42 on the information below. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time. From the phase. Heating Curve Questions And Answers.
From www.chegg.com
Solved Page 7 Name Heating Curve Worksheet The diagram Heating Curve Questions And Answers I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. It represents the heating of substance x at a constant rate of. Use the heating curve to explain why the temperature did not increase during state changes. From the phase diagram for water, determine the state of water at: Base. Heating Curve Questions And Answers.
From www.chegg.com
Solved You will use the following heating curve to answer Heating Curve Questions And Answers The heating curve shown above is a plot of temperature vs time. From the phase diagram for water, determine the state of water at: Answer sheet answer the following using the above heating curve 1. Evaporation of sweat requires energy and thus take excess heat away from. Use the heating curve to explain why the temperature did not increase during. Heating Curve Questions And Answers.
From wordworksheet.com
Heating And Cooling Curves Worksheet Heating Curve Questions And Answers Evaporation of sweat requires energy and thus take excess heat away from. The heating curve shown above is a plot of temperature vs time. From the phase diagram for water, determine the state of water at: Starting as a gas at 206°c, a sample of a substance is allowed to cool for. Use the heating curve to explain why the. Heating Curve Questions And Answers.
From www.numerade.com
SOLVED Use the heating curve for acetic acid to answer the following Heating Curve Questions And Answers Base your answers to questions 40 through 42 on the information below. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. What is the melting temperature of the above substance? Answer sheet answer the following using the above heating curve 1. The heating curve shown above is a. Heating Curve Questions And Answers.
From www.chegg.com
Solved Q5 Heating Curve 2 Points Consider the following Heating Curve Questions And Answers What is the melting temperature of the above substance? It represents the heating of substance x at a constant rate of. From the phase diagram for water, determine the state of water at: The heating curve shown above is a plot of temperature vs time. Base your answers to questions 40 through 42 on the information below. Use the heating. Heating Curve Questions And Answers.
From www.scribd.com
82218 Heating and Cooling Curve Answers PDF Heating Curve Questions And Answers What is the melting temperature of the above substance? Base your answers to questions 40 through 42 on the information below. Practice the experiment before you. From the phase diagram for water, determine the state of water at: I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. It represents. Heating Curve Questions And Answers.
From worksheets.clipart-library.com
Chemistry Heating Curve Worksheet Solution Exercises Chemistry Heating Curve Questions And Answers If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Base your answers to questions 40 through 42 on the information below. Evaporation of sweat requires energy and thus take excess heat away from. The heating curve shown above is a plot of temperature vs time. I can explain. Heating Curve Questions And Answers.
From studyfinder.org
The Ultimate Guide to Understanding Worksheet 1 Heating and Cooling Heating Curve Questions And Answers Answer sheet answer the following using the above heating curve 1. The heating curve shown above is a plot of temperature vs time. What is the melting temperature of the above substance? I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Practice the experiment before you. Starting as a. Heating Curve Questions And Answers.
From www.chegg.com
Solved A cooling curve is similar to a heating curve except Heating Curve Questions And Answers If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. What is the melting temperature of the above substance? Use the heating curve to explain why the temperature did not increase during state changes. Practice the experiment before you. Evaporation of sweat requires energy and thus take excess heat. Heating Curve Questions And Answers.
From www.chegg.com
Solved QUESTION 2 The heating curve for an unknown substance Heating Curve Questions And Answers Starting as a gas at 206°c, a sample of a substance is allowed to cool for. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Base your answers to questions 40 through 42 on the information below. Use the heating curve to explain why the temperature did not increase. Heating Curve Questions And Answers.
From studylib.net
heating curve worksheet Heating Curve Questions And Answers Base your answers to questions 40 through 42 on the information below. From the phase diagram for water, determine the state of water at: The heating curve shown above is a plot of temperature vs time. Evaporation of sweat requires energy and thus take excess heat away from. Practice the experiment before you. Use the heating curve to explain why. Heating Curve Questions And Answers.
From worksheets.clipart-library.com
Heating Curves Worksheet Freezing, Boiling, and Melting Points Heating Curve Questions And Answers From the phase diagram for water, determine the state of water at: I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Answer sheet answer the following using the above heating curve 1. It represents the heating of substance x at a constant rate of. Evaporation of sweat requires energy. Heating Curve Questions And Answers.
From studylib.net
A.2 Heat Curves Phase diagram Worksheet Key Heating Curve Questions And Answers Use the heating curve to explain why the temperature did not increase during state changes. Answer sheet answer the following using the above heating curve 1. Practice the experiment before you. It represents the heating of substance x at a constant rate of. Base your answers to questions 40 through 42 on the information below. The heating curve shown above. Heating Curve Questions And Answers.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve Questions And Answers Practice the experiment before you. The heating curve shown above is a plot of temperature vs time. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. Use the heating curve to explain why the temperature did not increase during state changes. What is the melting temperature of the. Heating Curve Questions And Answers.
From www.chegg.com
Solved d. Examine the heating curve for water below. Answer Heating Curve Questions And Answers Starting as a gas at 206°c, a sample of a substance is allowed to cool for. If a substance is heated and the temperature recorded over time, we can use the data to plot a heating curve. From the phase diagram for water, determine the state of water at: Answer sheet answer the following using the above heating curve 1.. Heating Curve Questions And Answers.
From worksheetdbtrommler.z19.web.core.windows.net
Heating And Cooling Curves Worksheet Answers Heating Curve Questions And Answers The heating curve shown above is a plot of temperature vs time. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Use the heating curve to explain why the temperature did not increase during state changes. Answer sheet answer the following using the above heating curve 1. It represents. Heating Curve Questions And Answers.
From www.numerade.com
SOLVED Emily Jhygeithi VII Heating Curves and Calorimetry Worksheet Heating Curve Questions And Answers Starting as a gas at 206°c, a sample of a substance is allowed to cool for. Base your answers to questions 40 through 42 on the information below. Answer sheet answer the following using the above heating curve 1. Evaporation of sweat requires energy and thus take excess heat away from. I can explain the shape of a heating/cooling curve. Heating Curve Questions And Answers.
From www.chegg.com
Solved For Review 432e 98. Use the heatingcooling curve Heating Curve Questions And Answers What is the melting temperature of the above substance? Base your answers to questions 40 through 42 on the information below. Evaporation of sweat requires energy and thus take excess heat away from. Use the heating curve to explain why the temperature did not increase during state changes. Starting as a gas at 206°c, a sample of a substance is. Heating Curve Questions And Answers.
From novenalunasolitaria.blogspot.com
Heating Cooling Curve Worksheet Answer Key worksheet Heating Curve Questions And Answers I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. Evaporation of sweat requires energy and thus take excess heat away from. From the phase diagram for water, determine the state of water at: Practice the experiment before you. If a substance is heated and the temperature recorded over time,. Heating Curve Questions And Answers.
From www.docsity.com
Heating Curve Worksheet 15 Questions Exercises Thermodynamics Docsity Heating Curve Questions And Answers Practice the experiment before you. The heating curve shown above is a plot of temperature vs time. I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance. What is the melting temperature of the above substance? Answer sheet answer the following using the above heating curve 1. If a substance. Heating Curve Questions And Answers.