Calorimeter Enthalpy Combustion . Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. The standard enthalpy of combustion \small. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. They can be calculated using a. to determine the enthalpy of combustion of fuels using a calorimeter. an overview of combustion calorimetry is presented in this paper. the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction. This process involves burning a. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol to. calculating enthalpy of reaction from combustion data. In section 5.6.3 we learned about bomb calorimetry. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry.
from exospxjed.blob.core.windows.net
enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. an overview of combustion calorimetry is presented in this paper. calculating enthalpy of reaction from combustion data. The standard enthalpy of combustion \small. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. In section 5.6.3 we learned about bomb calorimetry. the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. They can be calculated using a.
Calorimeter Constant at Wayne Mike blog
Calorimeter Enthalpy Combustion enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the. They can be calculated using a. enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry. calculating enthalpy of reaction from combustion data. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction. The standard enthalpy of combustion \small. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. an overview of combustion calorimetry is presented in this paper. This process involves burning a. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion.
From grade12uchemistry.weebly.com
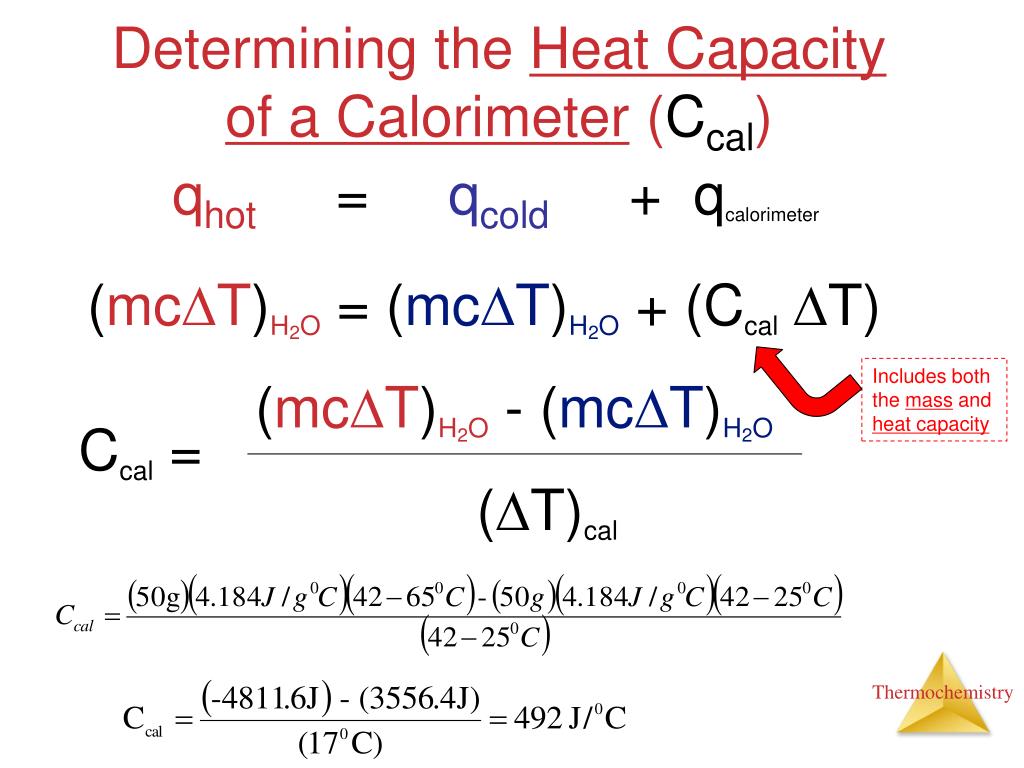
Calorimetry Grade12UChemistry Calorimeter Enthalpy Combustion revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. This process involves burning a. in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol to. calorimetry is used to measure quantities of. Calorimeter Enthalpy Combustion.
From exosbjzsg.blob.core.windows.net
Standard Enthalpy Of Formation Al2O3 at Lois Woodburn blog Calorimeter Enthalpy Combustion the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. in this experiment, you'll be measuring the heat of combustion of a series of five primary. Calorimeter Enthalpy Combustion.
From www.numerade.com
SOLVED Calculate the enthalpy of formation of sucrose [C12H22O11 Calorimeter Enthalpy Combustion Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. In section 5.6.3 we learned about bomb calorimetry. The standard enthalpy of combustion \small. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry. to determine the enthalpy of combustion of fuels using a calorimeter.. Calorimeter Enthalpy Combustion.
From www.numerade.com
SOLVED A bomb calorimeter can be used to measure the enthalpy of Calorimeter Enthalpy Combustion revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. enthalpies of combustion can be measured by burning a known amount of material. Calorimeter Enthalpy Combustion.
From www.youtube.com
Combustion calorimeter/ enthalpy / year 1 / OCR A chemistry YouTube Calorimeter Enthalpy Combustion In section 5.6.3 we learned about bomb calorimetry. calculating enthalpy of reaction from combustion data. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. They can be calculated using a. the. Calorimeter Enthalpy Combustion.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Calorimeter Enthalpy Combustion the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry. The standard enthalpy of combustion \small. in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol. Calorimeter Enthalpy Combustion.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 Calorimeter Enthalpy Combustion They can be calculated using a. to determine the enthalpy of combustion of fuels using a calorimeter. enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. 84 rows. Calorimeter Enthalpy Combustion.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Calorimeter Enthalpy Combustion This process involves burning a. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol to. calculating enthalpy of reaction from combustion data. calorimetry is used to. Calorimeter Enthalpy Combustion.
From www.numerade.com
SOLVED A student designed a calorimetry experiment to find the molar Calorimeter Enthalpy Combustion The standard enthalpy of combustion \small. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. They can be calculated using a. to determine the enthalpy of combustion of fuels using a calorimeter. enthalpies of combustion can be measured by burning a known amount of material in. Calorimeter Enthalpy Combustion.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimeter Enthalpy Combustion the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction. This process involves burning a. calculating enthalpy of reaction from combustion data. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. in this. Calorimeter Enthalpy Combustion.
From dxoqgiaho.blob.core.windows.net
Bomb Calorimeter Enthalpy Of Combustion at Harold Shaner blog Calorimeter Enthalpy Combustion They can be calculated using a. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the. an overview of combustion calorimetry is presented in this paper. the. Calorimeter Enthalpy Combustion.
From socratic.org
Finding DeltaH given heat released and mass in bomb calorimetry? Socratic Calorimeter Enthalpy Combustion revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. The standard enthalpy of combustion \small. They can be calculated using a. calorimetry. Calorimeter Enthalpy Combustion.
From ar.inspiredpencil.com
Heat Of Combustion Lab Calorimeter Enthalpy Combustion In section 5.6.3 we learned about bomb calorimetry. to determine the enthalpy of combustion of fuels using a calorimeter. an overview of combustion calorimetry is presented in this paper. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. calculating enthalpy of reaction from combustion data. This process involves. Calorimeter Enthalpy Combustion.
From www.youtube.com
How to calculate the enthalpy of combustion from experimental data Calorimeter Enthalpy Combustion calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. to determine the enthalpy of combustion of fuels using a calorimeter. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. enthalpies of combustion can be used to compare. Calorimeter Enthalpy Combustion.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Calorimeter Enthalpy Combustion use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. Heat of combustion. Calorimeter Enthalpy Combustion.
From saylordotorg.github.io
Calorimetry Calorimeter Enthalpy Combustion 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments.. Calorimeter Enthalpy Combustion.
From www.youtube.com
Enthalpy of Combustion Lab Explained YouTube Calorimeter Enthalpy Combustion revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. to determine the enthalpy of combustion of fuels using a calorimeter. This process involves burning a. Examples of applications of this method to the study. enthalpies of combustion can be used to. Calorimeter Enthalpy Combustion.
From learningzonegregorin2m.z4.web.core.windows.net
Heat Of Formation Formula Calorimeter Enthalpy Combustion In section 5.6.3 we learned about bomb calorimetry. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. calculating enthalpy of reaction from. Calorimeter Enthalpy Combustion.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Enthalpy Combustion enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the. enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. calorimetry is used to measure quantities of heat, and can be used to determine the heat of. Calorimeter Enthalpy Combustion.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Calorimeter Enthalpy Combustion bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. enthalpies of combustion can. Calorimeter Enthalpy Combustion.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimeter Enthalpy Combustion calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. Examples of applications of this method to the study. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The standard enthalpy of. Calorimeter Enthalpy Combustion.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimeter Enthalpy Combustion Examples of applications of this method to the study. the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. In section 5.6.3 we learned about bomb calorimetry. calculating enthalpy of reaction from combustion data. This process involves burning a. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions. Calorimeter Enthalpy Combustion.
From www.toppr.com
At 298K , the standard enthalpy of combustion of sucrose is 5737kJ Calorimeter Enthalpy Combustion This process involves burning a. They can be calculated using a. In section 5.6.3 we learned about bomb calorimetry. 84 rows there are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. the calorimeter and. Calorimeter Enthalpy Combustion.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Calorimeter Enthalpy Combustion Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. In section 5.6.3 we learned about bomb calorimetry. the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction.. Calorimeter Enthalpy Combustion.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Calorimeter Enthalpy Combustion to determine the enthalpy of combustion of fuels using a calorimeter. in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol to. an overview of combustion calorimetry is presented in this paper. enthalpies of combustion can be measured by burning a known amount of material in a. Calorimeter Enthalpy Combustion.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Calorimeter Enthalpy Combustion bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. They can be calculated using a. in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol to. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry. . Calorimeter Enthalpy Combustion.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Calorimeter Enthalpy Combustion Examples of applications of this method to the study. to determine the enthalpy of combustion of fuels using a calorimeter. use the heat capacity of the calorimeter, calculated in (a), to calculate (i) the molar internal energy of combustion,. They can be calculated using a. revision notes on 4.1.3 measurement of an enthalpy change for the aqa. Calorimeter Enthalpy Combustion.
From www.mdpi.com
Processes Free FullText Predicting Enthalpy of Combustion Using Calorimeter Enthalpy Combustion to determine the enthalpy of combustion of fuels using a calorimeter. They can be calculated using a. Examples of applications of this method to the study. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. in this experiment, you'll be measuring the heat of combustion of. Calorimeter Enthalpy Combustion.
From www.savemyexams.com
Determination of Enthalpy Changes OCR A Level Chemistry Revision Calorimeter Enthalpy Combustion the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction. This process involves burning a. the calorimeter and not the alcohol enthalpies of combustion can be calculated by using calorimetry. enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the.. Calorimeter Enthalpy Combustion.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Enthalpy Combustion in this experiment, you'll be measuring the heat of combustion of a series of five primary alcohols, from methanol to. bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. an overview of combustion calorimetry is presented in this paper. revision notes on 4.1.3 measurement of an enthalpy change for the. Calorimeter Enthalpy Combustion.
From dokumen.tips
(PDF) Rotatingbomb combustion calorimetry and the standard enthalpies Calorimeter Enthalpy Combustion bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. to determine the enthalpy of combustion of fuels using a calorimeter. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. the enthalpy of combustion can be calculated from the internal. Calorimeter Enthalpy Combustion.
From www.slideserve.com
PPT M olar enthalpy of combustion PowerPoint Presentation, free Calorimeter Enthalpy Combustion enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. in this experiment, you'll be measuring the heat of combustion of a. Calorimeter Enthalpy Combustion.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog Calorimeter Enthalpy Combustion the enthalpy of combustion can be calculated from the internal energy change if the balanced chemical reaction. The standard enthalpy of combustion \small. the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. enthalpies of combustion can be used to compare which fuels or substances release the most energy when they. Calorimeter Enthalpy Combustion.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Enthalpy Combustion Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry. Calorimeter Enthalpy Combustion.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimeter Enthalpy Combustion the energy changes that occur during reactions between solids or liquids with gases, particularly combustion reactions. enthalpies of combustion can be measured by burning a known amount of material in a bomb calorimeter and determining the. Heat of combustion of naphthalene most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. the calorimeter and. Calorimeter Enthalpy Combustion.