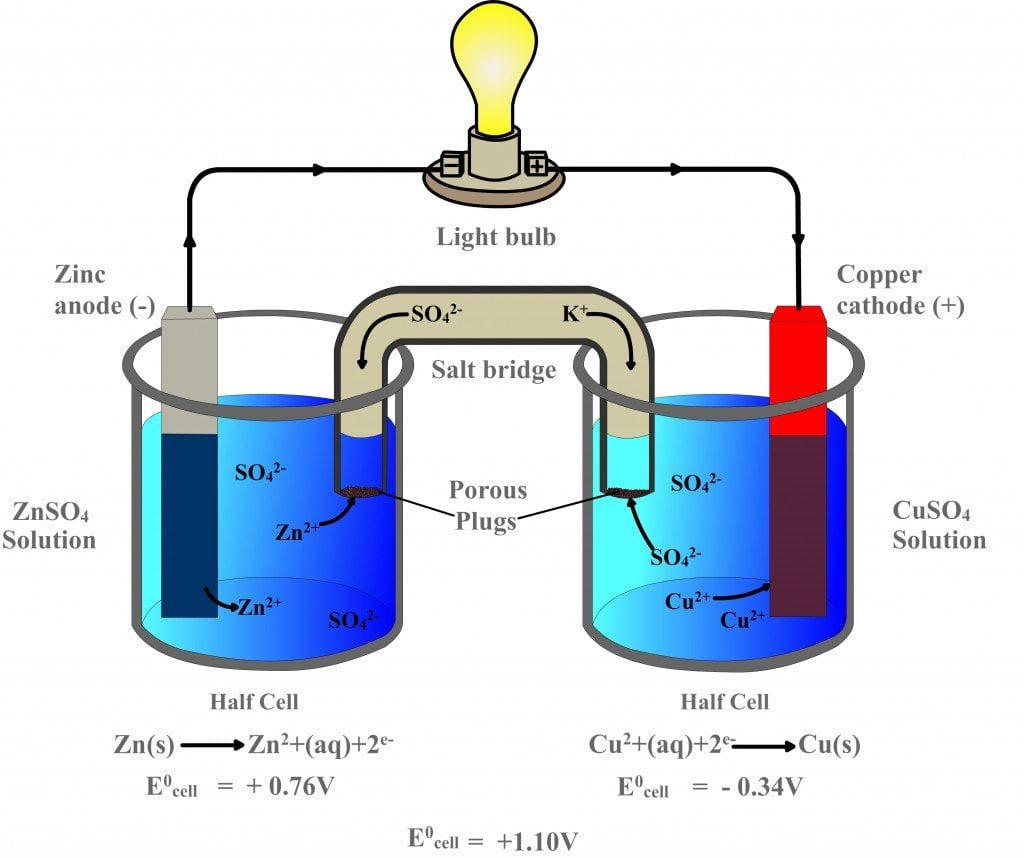
Standard Cell Potential Of Galvanic Cell . The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The potential difference is caused by the ability of. As a bonus, sketch a voltaic cell, label the anode and cathode, and. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Identify the oxidizing and reducing agents. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. What is the standard potential of the galvanic cell shown in figure 17.3?
from www.scienceabc.com
A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. The potential difference is caused by the ability of. What is the standard potential of the galvanic cell shown in figure 17.3? Identify the oxidizing and reducing agents. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. As a bonus, sketch a voltaic cell, label the anode and cathode, and. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of.
Galvanic Cell Definition, Diagram And Working
Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. Identify the oxidizing and reducing agents. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The potential difference is caused by the ability of. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. As a bonus, sketch a voltaic cell, label the anode and cathode, and. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. What is the standard potential of the galvanic cell shown in figure 17.3?
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Cell Potential Of Galvanic Cell As a bonus, sketch a voltaic cell, label the anode and cathode, and. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. The potential difference is caused by the ability of. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the. Standard Cell Potential Of Galvanic Cell.
From www.chegg.com
Solved A galvanic cell using Au+3/Au and Zn2+ / Zn was set Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The potential difference is caused by the ability of. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by. Standard Cell Potential Of Galvanic Cell.
From answerhappy.com
41. The standard cell potential of a galvanic cell based on the Standard Cell Potential Of Galvanic Cell As a bonus, sketch a voltaic cell, label the anode and cathode, and. Identify the oxidizing and reducing agents. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. For example,. Standard Cell Potential Of Galvanic Cell.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Standard Cell Potential Of Galvanic Cell The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Identify the oxidizing and reducing agents. The potential difference is caused by the ability of. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. The cell in figure. Standard Cell Potential Of Galvanic Cell.
From www.toppr.com
Calculate the standard cell potentials of galvanic cell in which the Standard Cell Potential Of Galvanic Cell A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. The potential difference is caused by the ability of. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. A galvanic cell can be constructed from a zinc electrode immersed. Standard Cell Potential Of Galvanic Cell.
From www.chegg.com
Solved The cell potential for the standard galvanic cell Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. Identify the oxidizing. Standard Cell Potential Of Galvanic Cell.
From www.chegg.com
Solved A galvanic cell using Au+/ Au and Ni2+/Ni was set up Standard Cell Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. Identify the oxidizing and reducing agents. For example, calculate the cell potential of the. Standard Cell Potential Of Galvanic Cell.
From www.chegg.com
Solved Use the halfreactions below to produce a voltaic Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. The potential difference is caused by the ability of. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. As a bonus, sketch a voltaic cell, label the anode and cathode, and. For example, calculate the cell potential of the galvanic cell. Standard Cell Potential Of Galvanic Cell.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components.. Standard Cell Potential Of Galvanic Cell.
From chem.libretexts.org
19.5 Standard Electrochemical Potentials Chemistry LibreTexts Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. As a bonus, sketch a voltaic cell, label the anode and cathode, and. Identify the oxidizing and reducing agents. A galvanic cell. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Cell Potential Of Galvanic Cell The potential difference is caused by the ability of. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. As a bonus, sketch a voltaic cell, label the anode and cathode, and. The cell potential, \(e_{cell}\), is the measure of the potential difference between. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
Galvanic cell potentials YouTube Standard Cell Potential Of Galvanic Cell What is the standard potential of the galvanic cell shown in figure 17.3? A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The potential difference is caused by the ability of. In this explainer, we will learn how to calculate the standard cell. Standard Cell Potential Of Galvanic Cell.
From www.numerade.com
SOLVED A certain halfreaction has standard reduction potential E" 0. Standard Cell Potential Of Galvanic Cell A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. The potential difference is caused by the ability of. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. For example, calculate the cell potential of the galvanic. Standard Cell Potential Of Galvanic Cell.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. As a bonus, sketch a voltaic cell, label the anode and cathode, and. The potential difference is caused by the ability of. A galvanic cell can be constructed from a zinc electrode. Standard Cell Potential Of Galvanic Cell.
From www.coursehero.com
[Solved] 1. Calculate the standard cell potential for the galvanic cell Standard Cell Potential Of Galvanic Cell The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt. Standard Cell Potential Of Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Standard Cell Potential Of Galvanic Cell The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential difference is caused by the ability of. As a bonus, sketch a voltaic cell, label the anode and cathode, and. Identify the oxidizing and reducing agents. A galvanic cell with a measured standard cell potential of 0.27 v is. Standard Cell Potential Of Galvanic Cell.
From www.numerade.com
SOLVED (8 points) Write the balanced equation for the galvanic cell Standard Cell Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. What is the standard potential of the galvanic cell shown in figure 17.3? A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
Galvanic Cell, Cell Notation, and Standard Cell Potential YouTube Standard Cell Potential Of Galvanic Cell In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Identify the oxidizing and reducing agents. The potential difference is caused by the ability of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The cell in figure. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode. Standard Cell Potential Of Galvanic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components.. Standard Cell Potential Of Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Standard Cell Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Identify the oxidizing and reducing agents. In this explainer, we will learn how to calculate. Standard Cell Potential Of Galvanic Cell.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. What is the standard potential of the galvanic cell shown in figure 17.3? A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode. Standard Cell Potential Of Galvanic Cell.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
Calculate the standard cell potentials of galvanic cell in whiCHM the Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components.. Standard Cell Potential Of Galvanic Cell.
From www.toppr.com
What will be standard cell potential of galvanic cell with the Standard Cell Potential Of Galvanic Cell The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed. Standard Cell Potential Of Galvanic Cell.
From askfilo.com
14. Calculate the standard cell potential of the galvanic cell in which t.. Standard Cell Potential Of Galvanic Cell The potential difference is caused by the ability of. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The cell in figure 17.3 is galvanic, the spontaneous cell. Standard Cell Potential Of Galvanic Cell.
From www.numerade.com
SOLVED (i) What is a cell potential? For each of the following Standard Cell Potential Of Galvanic Cell As a bonus, sketch a voltaic cell, label the anode and cathode, and. The cell in figure 17.3 is galvanic, the spontaneous cell reaction involving oxidation of its copper anode and reduction of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. A galvanic cell can be constructed from a. Standard Cell Potential Of Galvanic Cell.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Standard Cell Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. As a bonus, sketch a voltaic cell, label the anode and cathode, and. What is the standard potential of the galvanic cell shown in figure 17.3? In this explainer, we will learn how to. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
Calculatethe standard cell potentials of galvanic cell in which the Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. As a bonus, sketch a voltaic cell, label the anode and cathode, and. What is the standard potential of the galvanic cell shown in figure 17.3? A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The cell. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
The standard cell potential of the galvanic cell \( \mathrm{Zn Standard Cell Potential Of Galvanic Cell A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. As a bonus, sketch a voltaic cell, label the anode and cathode, and. The potential difference is caused by the ability of. Identify the oxidizing and reducing agents. A galvanic cell can be constructed from a zinc electrode. Standard Cell Potential Of Galvanic Cell.
From www.numerade.com
SOLVEDWhat is the standard cell potential for the voltaic (galvanic Standard Cell Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. A galvanic cell with a measured standard cell potential of 0.27 v. Standard Cell Potential Of Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Standard Cell Potential Of Galvanic Cell Identify the oxidizing and reducing agents. A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. The potential difference is caused by the ability of. In this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. A. Standard Cell Potential Of Galvanic Cell.
From www.chegg.com
Solved The galvanic cell described by Zn(s) Zn2+ (aq) Standard Cell Potential Of Galvanic Cell As a bonus, sketch a voltaic cell, label the anode and cathode, and. For example, calculate the cell potential of the galvanic cell prepared with ni/ni 2+ and mn/mn 2+ components. The potential difference is caused by the ability of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. A. Standard Cell Potential Of Galvanic Cell.
From www.chegg.com
The standard potential for the following galvanic Standard Cell Potential Of Galvanic Cell A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an. Standard Cell Potential Of Galvanic Cell.
From www.youtube.com
Find the cell potential of a galvanic cell based on the following Standard Cell Potential Of Galvanic Cell A galvanic cell with a measured standard cell potential of 0.27 v is constructed using two beakers connected by a salt bridge. The potential difference is caused by the ability of. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The cell in figure 17.3 is galvanic, the spontaneous cell. Standard Cell Potential Of Galvanic Cell.