Tin Iv Sulfate Chemical Formula . Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). We will start with ionic compounds, then covalent and then acids. Sulfate compounds are salts or esters. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. To balance the charges, we need two sulfate. This represents the formula snf 2, which is more properly. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. For example, you may see the words stannous fluoride on a tube of toothpaste. We use the principle of charge. An older system of nomenclature for such cations is still widely used, however. Determine the chemical formula for tin(iv) sulfate:
from www.numerade.com
An older system of nomenclature for such cations is still widely used, however. We will start with ionic compounds, then covalent and then acids. We use the principle of charge. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. Sulfate compounds are salts or esters. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. Determine the chemical formula for tin(iv) sulfate: This represents the formula snf 2, which is more properly. To balance the charges, we need two sulfate. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).
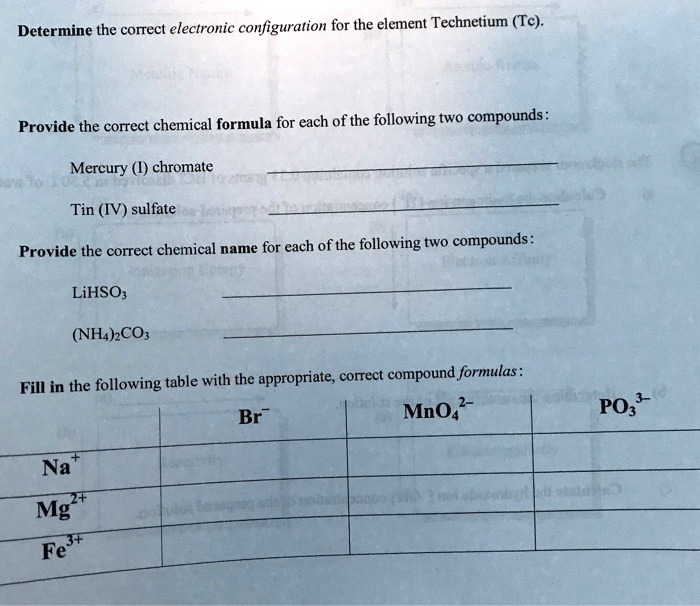
SOLVED Determine the correct electronic configuration for the element
Tin Iv Sulfate Chemical Formula For example, you may see the words stannous fluoride on a tube of toothpaste. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. For example, you may see the words stannous fluoride on a tube of toothpaste. To balance the charges, we need two sulfate. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Determine the chemical formula for tin(iv) sulfate: We use the principle of charge. This represents the formula snf 2, which is more properly. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. We will start with ionic compounds, then covalent and then acids. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. An older system of nomenclature for such cations is still widely used, however. Sulfate compounds are salts or esters.
From www.slideserve.com
PPT 1 st Semester Exam in High School Chemistry PowerPoint Tin Iv Sulfate Chemical Formula An older system of nomenclature for such cations is still widely used, however. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Determine the chemical formula for tin(iv) sulfate: The first step to finding the molar mass of tin(iv) sulfate is to count. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT Chemical Nomenclature Formula to Name PowerPoint Presentation Tin Iv Sulfate Chemical Formula In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. An older system of nomenclature for such cations is still widely used, however. To balance the charges, we need two sulfate. This represents the formula snf 2, which is more properly. We will start with ionic compounds, then covalent and then acids. Sulfate compounds. Tin Iv Sulfate Chemical Formula.
From slideplayer.com
Nomenclature Chapter ppt download Tin Iv Sulfate Chemical Formula We use the principle of charge. This represents the formula snf 2, which is more properly. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. An older system of nomenclature for such cations is still widely used, however. For example, you may see the words stannous fluoride on a tube. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds Tin Iv Sulfate Chemical Formula We will start with ionic compounds, then covalent and then acids. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. For example, you may see the words stannous fluoride on a tube of toothpaste. The first step to finding the molar mass of tin(iv) sulfate is to count the number. Tin Iv Sulfate Chemical Formula.
From www.youtube.com
How to Write the Formula for Tin (II) sulfate YouTube Tin Iv Sulfate Chemical Formula To balance the charges, we need two sulfate. This represents the formula snf 2, which is more properly. We will start with ionic compounds, then covalent and then acids. Sulfate compounds are salts or esters. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. Tin (iv) sulfate (stannic sulfate) is a moderately water. Tin Iv Sulfate Chemical Formula.
From www.numerade.com
SOLVEDWrite formulas for these compounds (a) tin (IV) bromide (d Tin Iv Sulfate Chemical Formula We use the principle of charge. We will start with ionic compounds, then covalent and then acids. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. For example, you may see the words stannous fluoride on a tube of toothpaste. The first step to finding the molar mass of tin(iv) sulfate is to. Tin Iv Sulfate Chemical Formula.
From www.youtube.com
How to Write the Name for Sn(SO4)2 YouTube Tin Iv Sulfate Chemical Formula We use the principle of charge. For example, you may see the words stannous fluoride on a tube of toothpaste. Sulfate compounds are salts or esters. This represents the formula snf 2, which is more properly. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule. Tin Iv Sulfate Chemical Formula.
From butchixanh.edu.vn
Top 7+ tin iv sulfide formula Latest Bút Chì Xanh Tin Iv Sulfate Chemical Formula We use the principle of charge. An older system of nomenclature for such cations is still widely used, however. To balance the charges, we need two sulfate. We will start with ionic compounds, then covalent and then acids. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and. Tin Iv Sulfate Chemical Formula.
From www.chegg.com
Solved 4010 Question 22 (1 point) Write the name for Tin Iv Sulfate Chemical Formula The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Tin (iv) sulfate (stannic sulfate) is a. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT Naming Ionic Compounds Wkst 1 PowerPoint Presentation ID1932060 Tin Iv Sulfate Chemical Formula The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. We will start with ionic compounds, then covalent and then acids. Sulfate compounds are salts or esters. To balance the charges, we need two sulfate. We use the principle of charge. An older. Tin Iv Sulfate Chemical Formula.
From www.toppr.com
the following 52. Write the formula compounds (A) Mercury (II Tin Iv Sulfate Chemical Formula Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. Determine the chemical formula for tin(iv) sulfate: We use the principle of charge. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. An older system of nomenclature for such cations is still widely used,. Tin Iv Sulfate Chemical Formula.
From slideplayer.com
Preview Lesson Starter Objectives Significance of a Chemical Formula Tin Iv Sulfate Chemical Formula An older system of nomenclature for such cations is still widely used, however. We use the principle of charge. We will start with ionic compounds, then covalent and then acids. This represents the formula snf 2, which is more properly. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. For example, you may. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT Chemical Nomenclature Formula to Name PowerPoint Presentation Tin Iv Sulfate Chemical Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). This represents the formula snf 2, which is more properly. An older system of nomenclature for such cations is still widely used, however. Sulfate compounds are salts or esters. Tin (iv) sulfate (stannic sulfate). Tin Iv Sulfate Chemical Formula.
From www.fishersci.de
Cer(IV)sulfatLösung, 0,1 M Ce(SO4)2, Honeywell Fisher Scientific Tin Iv Sulfate Chemical Formula In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. An older system of nomenclature for such cations is still widely used, however. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. This represents the formula snf 2, which is more properly. We will. Tin Iv Sulfate Chemical Formula.
From www.youtube.com
how to write chemical formula of Tin (IV) SulfateMolecular formula Tin Tin Iv Sulfate Chemical Formula Determine the chemical formula for tin(iv) sulfate: Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Sulfate compounds are salts or esters. For example,. Tin Iv Sulfate Chemical Formula.
From www.coursehero.com
[Solved] Please help. 16. What is the correct chemical formula of tin Tin Iv Sulfate Chemical Formula In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. Determine the chemical formula for tin(iv) sulfate: To balance the charges, we need two sulfate. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. Sulfate compounds. Tin Iv Sulfate Chemical Formula.
From testbook.com
Tin (IV) Chloride Formula Know Structure, Properties, and Uses Tin Iv Sulfate Chemical Formula To balance the charges, we need two sulfate. An older system of nomenclature for such cations is still widely used, however. Sulfate compounds are salts or esters. Determine the chemical formula for tin(iv) sulfate: This represents the formula snf 2, which is more properly. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is. Tin Iv Sulfate Chemical Formula.
From www.kaskus.co.id
Mengenal Lebih Dekat Sulfat Dalam Perspektif Kimia Lingkungan KASKUS Tin Iv Sulfate Chemical Formula For example, you may see the words stannous fluoride on a tube of toothpaste. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Sulfate compounds are salts or esters. We will start with ionic compounds, then covalent and then acids. Determine the chemical. Tin Iv Sulfate Chemical Formula.
From www.youtube.com
How to Write the Formula for Tin (IV) sulfate YouTube Tin Iv Sulfate Chemical Formula Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. For example, you may see the words stannous fluoride on a tube of toothpaste. An older system of nomenclature for such cations is still widely. Tin Iv Sulfate Chemical Formula.
From www.coursehero.com
[Solved] . 1. Write the balanced molecular chemical equation for the Tin Iv Sulfate Chemical Formula To balance the charges, we need two sulfate. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). We will start with ionic compounds, then covalent and then acids. Sulfate compounds are salts or esters. For example, you may see the words stannous fluoride. Tin Iv Sulfate Chemical Formula.
From www.numerade.com
SOLVEDFor each compound, list the correct formula and calculate the Tin Iv Sulfate Chemical Formula Determine the chemical formula for tin(iv) sulfate: Sulfate compounds are salts or esters. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. An older system of. Tin Iv Sulfate Chemical Formula.
From www.numerade.com
SOLVED 3 Nickel 1 oxide formulas for the following compounds. Iron Tin Iv Sulfate Chemical Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). We will start with ionic compounds, then covalent and then acids. An older system of nomenclature for such cations is still widely used, however. Tin (iv) sulfate (stannic sulfate) is a moderately water and. Tin Iv Sulfate Chemical Formula.
From www.chegg.com
Solved Formulas Type II Ternary 1) tin(IV) chlorite 3) Tin Iv Sulfate Chemical Formula We will start with ionic compounds, then covalent and then acids. An older system of nomenclature for such cations is still widely used, however. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. Sulfate compounds are salts or esters. To balance the. Tin Iv Sulfate Chemical Formula.
From www.fishersci.com
Sodium tin(IV) oxide hydrate, Reagent Grade, Thermo Scientific Tin Iv Sulfate Chemical Formula We will start with ionic compounds, then covalent and then acids. This represents the formula snf 2, which is more properly. Sulfate compounds are salts or esters. An older system of nomenclature for such cations is still widely used, however. For example, you may see the words stannous fluoride on a tube of toothpaste. In this video we'll write the. Tin Iv Sulfate Chemical Formula.
From www.numerade.com
SOLVED Determine the correct electronic configuration for the element Tin Iv Sulfate Chemical Formula To balance the charges, we need two sulfate. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. For example, you may see the words stannous fluoride on a tube of toothpaste. Sulfate compounds are salts or esters. An older system of nomenclature for such cations is still widely used, however. Tin (iv) sulfate. Tin Iv Sulfate Chemical Formula.
From www.numerade.com
SOLVEDWrite formulas for the following compounds. a. Sulfur dioxide h Tin Iv Sulfate Chemical Formula Determine the chemical formula for tin(iv) sulfate: Sulfate compounds are salts or esters. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula snf 2, which is more properly. In this video we'll write the correct formula for tin (iv) sulfate (sn (so4)2).to write the. We will start with ionic compounds, then. Tin Iv Sulfate Chemical Formula.
From www.shutterstock.com
Tin Iv Oxide Images Browse 4 Stock Photos & Vectors Free Download with Tin Iv Sulfate Chemical Formula This represents the formula snf 2, which is more properly. An older system of nomenclature for such cations is still widely used, however. We use the principle of charge. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Determine the chemical formula for. Tin Iv Sulfate Chemical Formula.
From slideplayer.com
Chapter 7 Naming Monatomic Ions Section 1 Chemical Names and Formulas Tin Iv Sulfate Chemical Formula Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. Determine the chemical formula for tin(iv) sulfate: We will start with ionic compounds, then. Tin Iv Sulfate Chemical Formula.
From www.chegg.com
Solved Exercise 5.97 Part A For the compound tin(IV) sulfate Tin Iv Sulfate Chemical Formula For example, you may see the words stannous fluoride on a tube of toothpaste. We will start with ionic compounds, then covalent and then acids. Determine the chemical formula for tin(iv) sulfate: Sulfate compounds are salts or esters. An older system of nomenclature for such cations is still widely used, however. Tin (iv) sulfate (stannic sulfate) is a moderately water. Tin Iv Sulfate Chemical Formula.
From www.fishersci.com
Tin(IV) oxide, 15 in H{2}O colloidal dispersion, Thermo Scientific Tin Iv Sulfate Chemical Formula We will start with ionic compounds, then covalent and then acids. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. An older system of. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT Chapter 9 Naming Compounds and Writing Formulas PowerPoint Tin Iv Sulfate Chemical Formula Determine the chemical formula for tin(iv) sulfate: For example, you may see the words stannous fluoride on a tube of toothpaste. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. This represents the formula snf 2, which is more properly. We will start with ionic compounds, then covalent and then. Tin Iv Sulfate Chemical Formula.
From www.toppr.com
2 Write the formula the following compounds A. Mercury(II) chloride B Tin Iv Sulfate Chemical Formula Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. We use the principle of charge. Determine the chemical formula for tin(iv) sulfate: We will start with ionic compounds, then covalent and then acids. Sulfate compounds are salts or esters. Thus cu + is copper(i) (read as “copper one”), fe 2+. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT Calcium Sulfide PowerPoint Presentation, free download ID3730769 Tin Iv Sulfate Chemical Formula The first step to finding the molar mass of tin(iv) sulfate is to count the number of each atom present in a single molecule using the chemical. We use the principle of charge. For example, you may see the words stannous fluoride on a tube of toothpaste. In this video we'll write the correct formula for tin (iv) sulfate (sn. Tin Iv Sulfate Chemical Formula.
From slideplayer.com
Chemical Nomenclature. Octet Rule n Atoms tend to achieve electron Tin Iv Sulfate Chemical Formula We use the principle of charge. Tin (iv) sulfate (stannic sulfate) is a moderately water and acid soluble tin source for uses compatible with sulfates. For example, you may see the words stannous fluoride on a tube of toothpaste. Sulfate compounds are salts or esters. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+. Tin Iv Sulfate Chemical Formula.
From www.slideserve.com
PPT How to Figure Out Chemical Formulas PowerPoint Presentation ID Tin Iv Sulfate Chemical Formula We will start with ionic compounds, then covalent and then acids. For example, you may see the words stannous fluoride on a tube of toothpaste. An older system of nomenclature for such cations is still widely used, however. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and. Tin Iv Sulfate Chemical Formula.