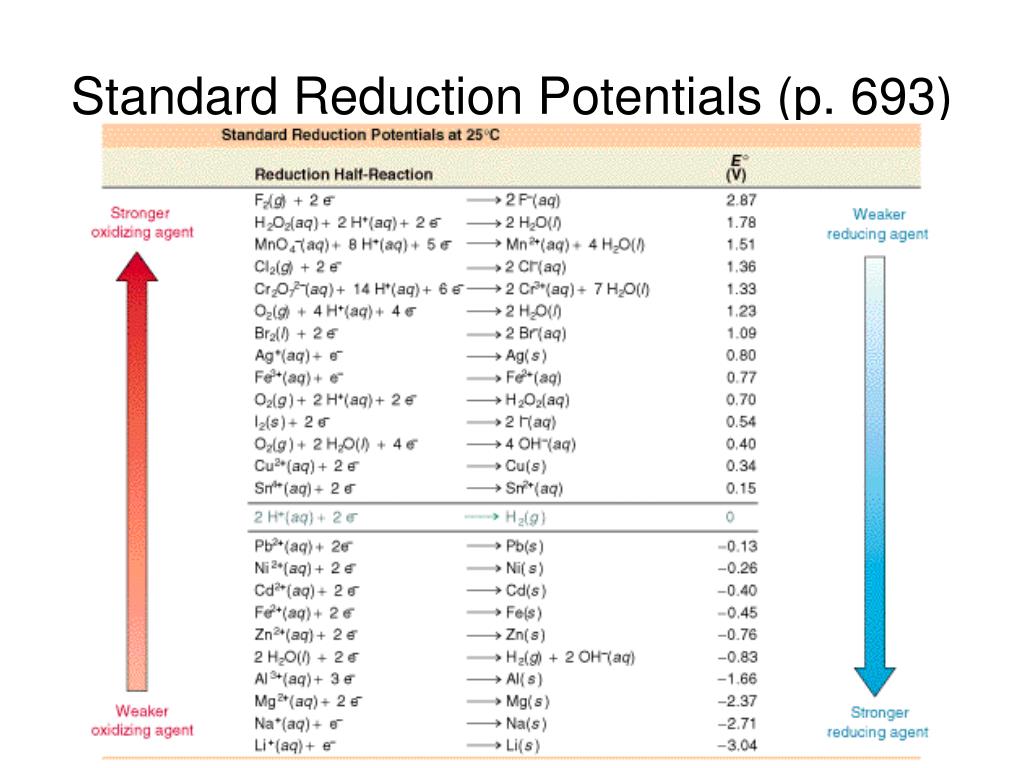
Standard Reduction Potential Negative . all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. The reaction at the anode will be the half. Use standard reduction potentials to determine the. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Note that elemental fluorine, at. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as.
from www.slideserve.com
conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. Note that elemental fluorine, at. Use standard reduction potentials to determine the. The reaction at the anode will be the half.
PPT Oxidation and Reduction PowerPoint Presentation, free download
Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The reaction at the anode will be the half. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Note that elemental fluorine, at. Use standard reduction potentials to determine the. galvanic cells have positive cell potentials, and all the reduction reactions are reversible.
From pubs.acs.org
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Standard Reduction Potential Negative Note that elemental fluorine, at. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. Use standard reduction potentials to determine the. all reactions are listed as reduction reactions, where an element or ion gains. Standard Reduction Potential Negative.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The reaction at the anode will be the half. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Note that elemental fluorine, at. . Standard Reduction Potential Negative.
From www.slideserve.com
PPT Redox reactions PowerPoint Presentation, free download ID3446507 Standard Reduction Potential Negative since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. Note that elemental fluorine, at. the standard reduction potential is the tendency for a chemical species to be reduced, and. Standard Reduction Potential Negative.
From slideplayer.com
Electrochemistry. ppt download Standard Reduction Potential Negative The reaction at the anode will be the half. Note that elemental fluorine, at. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. Use standard reduction potentials to determine the. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts. Standard Reduction Potential Negative.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570 Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. Note that elemental fluorine, at. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. since the tabulated standard electrode potentials are reduction potentials, the one which is. Standard Reduction Potential Negative.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Negative Use standard reduction potentials to determine the. The reaction at the anode will be the half. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. Note that elemental fluorine, at. the standard reduction potential is. Standard Reduction Potential Negative.
From askfilo.com
Explanation The negative value of standard reduction potential for Cr3+ Standard Reduction Potential Negative The reaction at the anode will be the half. Use standard reduction potentials to determine the. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Note that elemental fluorine, at. conversely, a more negative standard reduction potential indicates a lower tendency for the species to. Standard Reduction Potential Negative.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Negative all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Use standard reduction potentials to determine the. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Electron Transport Chain PowerPoint Presentation, free download Standard Reduction Potential Negative Use standard reduction potentials to determine the. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. conversely, a more negative standard reduction potential indicates a. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID5404690 Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. Use standard reduction potentials to determine the. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. since the tabulated standard electrode potentials are reduction potentials, the one. Standard Reduction Potential Negative.
From www.chegg.com
Solved Use the standard reduction potentials located in the Standard Reduction Potential Negative all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. The reaction at the anode will be the half. Use standard reduction potentials to determine the. the standard. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. conversely, a more negative standard reduction potential indicates a lower tendency for the species. Standard Reduction Potential Negative.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Negative galvanic cells have positive cell potentials, and all the reduction reactions are reversible. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Note that elemental fluorine, at. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as.. Standard Reduction Potential Negative.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Negative all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Use standard reduction potentials to determine the. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be. Standard Reduction Potential Negative.
From www.toppr.com
The standard reduction potentials for two reactions are given below Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. since the tabulated. Standard Reduction Potential Negative.
From vpia.org.vn
Standard Reduction Potentials made easy, reduction line Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. The reaction at. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The reaction at the anode will be the half. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. galvanic cells have positive cell potentials, and all. Standard Reduction Potential Negative.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Negative since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. Note that elemental fluorine, at. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The reaction at the anode will be the half. all reactions. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Reduction Potential Negative The reaction at the anode will be the half. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Use standard reduction potentials to determine the. Note that elemental fluorine, at. the standard reduction potential is the tendency for a chemical species to be reduced, and is. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Electrochemistry & Virus Templated Electrodes PowerPoint Standard Reduction Potential Negative Use standard reduction potentials to determine the. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Note that elemental fluorine, at. The reaction at the anode will be the half. since the. Standard Reduction Potential Negative.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Negative The reaction at the anode will be the half. Note that elemental fluorine, at. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. Use standard reduction potentials to determine the. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. all reactions are listed as. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Electrochemistry Part III Reduction Potentials PowerPoint Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. Note that elemental fluorine, at. The reaction at the anode will be the half. Use standard reduction potentials to determine the. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be.. Standard Reduction Potential Negative.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Negative since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. all reactions. Standard Reduction Potential Negative.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential Negative The reaction at the anode will be the half. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. Note that elemental fluorine, at. Use standard reduction potentials to determine the. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be.. Standard Reduction Potential Negative.
From quizturbinates.z21.web.core.windows.net
Where Does Oxidation Occur Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. The reaction at the anode will be the half. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. galvanic cells have positive cell potentials, and all the. Standard Reduction Potential Negative.
From www.chegg.com
Solved TABLE 14.1 "Electron Tower" of Standard Reduction Standard Reduction Potential Negative Note that elemental fluorine, at. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. since the tabulated standard electrode potentials are reduction potentials, the one which is. Standard Reduction Potential Negative.
From saylordotorg.github.io
Standard Potentials Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The reaction at the anode will be the half. Use standard reduction potentials to determine the. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. . Standard Reduction Potential Negative.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Negative conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. The reaction at the anode will be the half. Use standard reduction potentials to determine the. all reactions are listed. Standard Reduction Potential Negative.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Reduction Potential Negative the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. conversely, a more negative standard reduction potential indicates a lower tendency for the species to be reduced, as. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need. Standard Reduction Potential Negative.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Standard Reduction Potential Negative all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured. Standard Reduction Potential Negative.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Negative Use standard reduction potentials to determine the. since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. The reaction at the anode will be the half. Note that elemental fluorine, at. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Negative.
From www.youtube.com
20.13 standard reduction potential problem YouTube Standard Reduction Potential Negative since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. Use standard reduction potentials to determine the. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. Note that elemental fluorine, at. The reaction at the anode will. Standard Reduction Potential Negative.
From www.thetechedvocate.org
How to Calculate Standard Reduction Potential The Tech Edvocate Standard Reduction Potential Negative since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Use standard reduction potentials to determine the. The reaction at the anode will be the half. . Standard Reduction Potential Negative.
From pveducation.org
Standard Potential PVEducation Standard Reduction Potential Negative galvanic cells have positive cell potentials, and all the reduction reactions are reversible. The reaction at the anode will be the half. all reactions are listed as reduction reactions, where an element or ion gains electrons and takes on a more negative charge. conversely, a more negative standard reduction potential indicates a lower tendency for the species. Standard Reduction Potential Negative.