Thermometers Reprocessing Requirements . Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by.
from learn.e-limu.org
To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessing instructions should indicate the appropriate microbicidal process for the device.
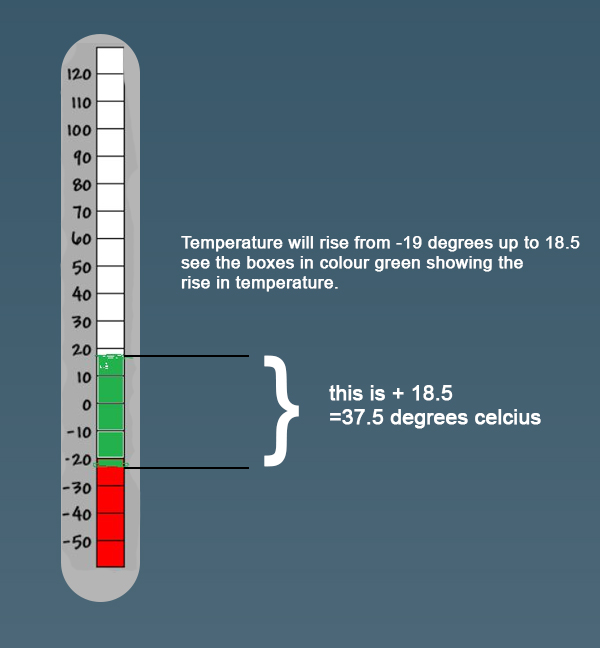
eLimu Temperature
Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be.
From www.dreamstime.com
Thermometers Different Levels Stock Vector Illustration of health Thermometers Reprocessing Requirements This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessing of reusable devices and equipment should be consistent with the current. Thermometers Reprocessing Requirements.
From ozstar.com.au
How Thermometers Work Ozstar Australia Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must. Thermometers Reprocessing Requirements.
From operonstrategist.com
Regulatory Requirements for Digital Thermometer Thermometers Reprocessing Requirements Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the. Thermometers Reprocessing Requirements.
From electricalworkbook.com
What is Digital Thermometer? Working, Block Diagram & Advantages Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessing of reusable devices. Thermometers Reprocessing Requirements.
From learn.e-limu.org
eLimu Temperature Thermometers Reprocessing Requirements Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. To avoid any risk of infection by a contaminated. Thermometers Reprocessing Requirements.
From instrumate.com
Superior thermometer 414 InstruMate Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must. Thermometers Reprocessing Requirements.
From cooking.stackexchange.com
equipment How should I read a thermometer when it's got an extra Thermometers Reprocessing Requirements Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the. Thermometers Reprocessing Requirements.
From www.alamy.com
Classic outdoor and indoor fahrenheit and celsius thermometers set for Thermometers Reprocessing Requirements To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to. Thermometers Reprocessing Requirements.
From www.alamy.com
Standard thermometers hires stock photography and images Alamy Thermometers Reprocessing Requirements Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes. Thermometers Reprocessing Requirements.
From www.chemicalslearning.com
Types of Thermometers and Working Principle and Advantages Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessors must be able to present objective evidence that all validation requirements. Thermometers Reprocessing Requirements.
From byjus.com
Identify the type of thermometers. Thermometers Reprocessing Requirements Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Medical equipment that is to be reprocessed must be labeled. Thermometers Reprocessing Requirements.
From www.scribd.com
Physics Notes on Types of Thermometers Mercury Thermometers Thermometers Reprocessing Requirements Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian. Thermometers Reprocessing Requirements.
From www.forestry-suppliers.com
Traceable Extreme Accuracy Standards Thermometer Forestry Suppliers, Inc. Thermometers Reprocessing Requirements Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for. Thermometers Reprocessing Requirements.
From manual.nutritionforlearning.ca
Thermometers Program Coordinator Resources Thermometers Reprocessing Requirements To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes. Thermometers Reprocessing Requirements.
From davida.davivienda.com
Reading Thermometers Worksheet Printable Word Searches Thermometers Reprocessing Requirements Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to. Thermometers Reprocessing Requirements.
From www.pinterest.com
food thermometers and temperature indicators are shown in this graphic Thermometers Reprocessing Requirements To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for. Thermometers Reprocessing Requirements.
From www.liveworksheets.com
Types of thermometers 3044 Nasir_Dollah72 Live Thermometers Reprocessing Requirements Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must. Thermometers Reprocessing Requirements.
From www.wikihow.com
How to Easily Change Your Thermometer from °C to °F Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. This guidance provides recommendations for the formulation and. Thermometers Reprocessing Requirements.
From studylib.net
Dial Thermometers Thermometers Reprocessing Requirements To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessors. Thermometers Reprocessing Requirements.
From citizenside.com
How To Calibrate A Thermometer Digital CitizenSide Thermometers Reprocessing Requirements Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must. Thermometers Reprocessing Requirements.
From www.sciencephoto.com
Thermometers Stock Image C007/8069 Science Photo Library Thermometers Reprocessing Requirements Reprocessing instructions should indicate the appropriate microbicidal process for the device. To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Health care organizations must ensure that. Thermometers Reprocessing Requirements.
From ubicaciondepersonas.cdmx.gob.mx
Thermometer Goal Chart ubicaciondepersonas.cdmx.gob.mx Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessors must be able. Thermometers Reprocessing Requirements.
From www.youtube.com
For the construction of a thermometer, one of the essential Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied. Thermometers Reprocessing Requirements.
From askanydifference.com
Laboratory Thermometer vs Clinical Thermometer Difference and Comparison Thermometers Reprocessing Requirements Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Reprocessing of. Thermometers Reprocessing Requirements.
From theinstrumentguru.com
Parts of Thermometer Digital Thermometer Thermometers Reprocessing Requirements Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to. Thermometers Reprocessing Requirements.
From pbrainmd.wordpress.com
Federal US probe reprocessing requirements by procedure Peripheral Brain Thermometers Reprocessing Requirements Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Health care. Thermometers Reprocessing Requirements.
From www.stocksy.com
«Medical Thermometers For Measuring Temperature.» del colaborador de Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and.. Thermometers Reprocessing Requirements.
From www.servotech.co.nz
Talk to Servotech experts on indicator thermometers and all Thermometers Reprocessing Requirements To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and.. Thermometers Reprocessing Requirements.
From www.researchgate.net
Adapted subjective units of distress thermometers Download Scientific Thermometers Reprocessing Requirements Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. This guidance provides recommendations for the. Thermometers Reprocessing Requirements.
From www.slideserve.com
PPT REPLACING MERCURY CONTAINING PRODUCTS AND MERCURY THERMOMETERS IN Thermometers Reprocessing Requirements Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Medical equipment that is to be reprocessed must be labeled reusable by the manufacturer and must be accompanied by. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. To avoid any. Thermometers Reprocessing Requirements.
From device.report
Thermometers ASAPD02 Infrared Thermometer Instruction Manual Thermometers Reprocessing Requirements Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the. Thermometers Reprocessing Requirements.
From www.embibe.com
Draw the diagram of a clinical thermometer and label its parts Thermometers Reprocessing Requirements This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Medical equipment that is to be reprocessed must be labeled. Thermometers Reprocessing Requirements.
From www.conrad.com
Thermometer TFA LT102 Temperature reading range 40 up to +70 °C Thermometers Reprocessing Requirements Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Reprocessing instructions should indicate the appropriate microbicidal process for the device. This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessors must be able to present objective evidence that all validation. Thermometers Reprocessing Requirements.
From www.teachingexpertise.com
19 Teachable Thermometer Activities For Kids Of All Ages Teaching Thermometers Reprocessing Requirements To avoid any risk of infection by a contaminated device, reusable devices undergo reprocessing, a detailed, multistep process to clean and then. Reprocessing of reusable devices and equipment should be consistent with the current edition of the australian guidelines for the prevention and. Reprocessing instructions should indicate the appropriate microbicidal process for the device. Reprocessors must be able to present. Thermometers Reprocessing Requirements.
From www.youtube.com
Clinical thermometer diagram thermometer drawing Class7 YouTube Thermometers Reprocessing Requirements This guidance provides recommendations for the formulation and scientific validation of reprocessing instructions for reusable medical devices. Reprocessors must be able to present objective evidence that all validation requirements have been met and that the processes which must be. Health care organizations must ensure that critical instruments and devices that are being reprocessed are intended to be reused and. To. Thermometers Reprocessing Requirements.