Copper Green Oxidation State . Copper (ii) complex ions, but there is also a substantial. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. This is what a reaction is called when atoms change their. For example, both [cu(nh 3 ). the chemistry of copper is dominated by the +2 oxidation state, e.g. The copper oxide will continue reacting to oxygen over time. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. copper will start to react with the oxygen in the air to form copper oxide. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state.
from chemistry.com.pk
The copper oxide will continue reacting to oxygen over time. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. the chemistry of copper is dominated by the +2 oxidation state, e.g. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. copper will start to react with the oxygen in the air to form copper oxide. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. This is what a reaction is called when atoms change their. Copper (ii) complex ions, but there is also a substantial. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air.
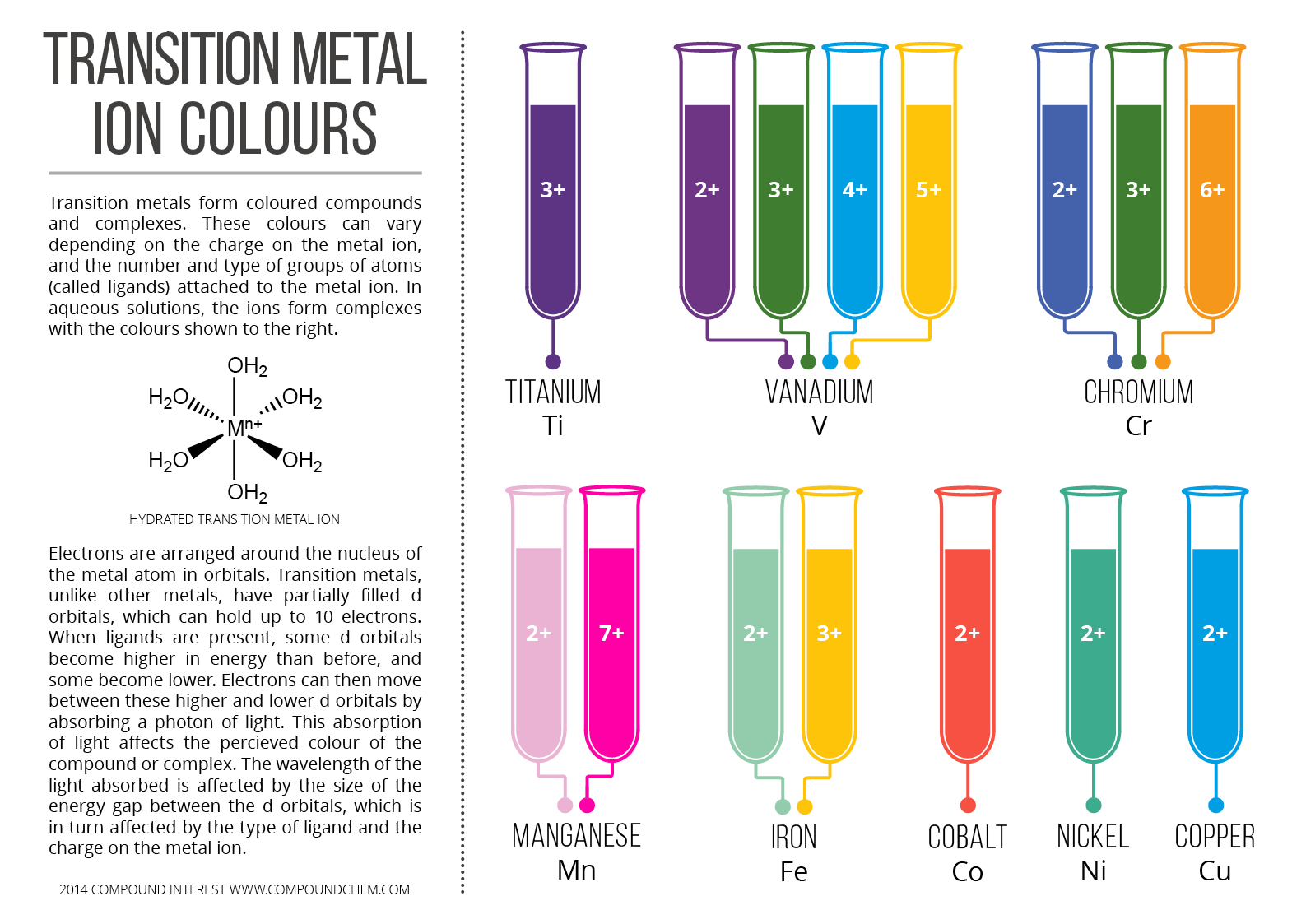
Colours of Transition Metal Ions in Aqueous Solution [Infographic
Copper Green Oxidation State Copper (ii) complex ions, but there is also a substantial. The copper oxide will continue reacting to oxygen over time. For example, both [cu(nh 3 ). the chemistry of copper is dominated by the +2 oxidation state, e.g. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. This is what a reaction is called when atoms change their. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. Copper (ii) complex ions, but there is also a substantial. copper will start to react with the oxygen in the air to form copper oxide. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air.
From happinessforever.net
Why Does Copper Turn Green Over Time? 10 Secret Facts About Statue Of Copper Green Oxidation State The copper oxide will continue reacting to oxygen over time. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. For example, both [cu(nh 3 ). copper will start to. Copper Green Oxidation State.
From pubs.rsc.org
Observing the oxidation state turnover in heterogeneous iridiumbased Copper Green Oxidation State This is what a reaction is called when atoms change their. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. Copper (ii) complex ions, but there is also a substantial. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. copper. Copper Green Oxidation State.
From melscience.com
Methods of copper oxidation MEL Chemistry Copper Green Oxidation State For example, both [cu(nh 3 ). This is what a reaction is called when atoms change their. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. the chemistry of copper is dominated by the +2 oxidation state, e.g. the vinegar mixture causes a chemical reaction between the copper. Copper Green Oxidation State.
From sciencenotes.org
Downloadable Periodic Table Oxidation States Copper Green Oxidation State The copper oxide will continue reacting to oxygen over time. For example, both [cu(nh 3 ). This is what a reaction is called when atoms change their. Copper (ii) complex ions, but there is also a substantial. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. copper has a beautiful. Copper Green Oxidation State.
From www.doubtnut.com
Transition metal which forms green compounds in its +3 oxidation state Copper Green Oxidation State the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. This is what a reaction is called when atoms change their. copper will start to react with the oxygen in the air to form copper oxide. forming copper(i) complexes (other than the one with water as a ligand) also stabalises. Copper Green Oxidation State.
From www.youtube.com
What is the oxidation state of each element in COH2? YouTube Copper Green Oxidation State the chemistry of copper is dominated by the +2 oxidation state, e.g. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. This is what a reaction is called when atoms change their. the vinegar mixture causes a chemical reaction between the copper and the air known as. Copper Green Oxidation State.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Copper Green Oxidation State forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. copper will start to react with the oxygen in the air to form copper oxide. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. the two principal oxidation. Copper Green Oxidation State.
From www.youtube.com
Variable oxidation states copper chemistry YouTube Copper Green Oxidation State copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. This is what a reaction is called when atoms change their. copper will start to react with the oxygen in the air to form copper oxide. Copper (ii) complex ions, but there is also a substantial. the two. Copper Green Oxidation State.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Copper Green Oxidation State the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. The copper oxide will continue reacting to oxygen over time. This is what a reaction is called when atoms change their. Copper (ii). Copper Green Oxidation State.
From www.chegg.com
Reagen Chart of Oxidation States for Common Compound Copper Green Oxidation State the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. the chemistry of copper is dominated by the +2 oxidation state, e.g. copper will start to react with the oxygen in the air to form copper oxide. This is what a reaction is called when atoms change their. copper. Copper Green Oxidation State.
From goodbeeplumbinganddrains.com
How Do You Remove Green Corrosion from Copper Pipes? GoodBee Plumbing Copper Green Oxidation State forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. This is what a reaction is called when atoms change their. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. the chemistry of copper is dominated by the +2 oxidation state,. Copper Green Oxidation State.
From sciencenotes.org
Transition Metal Ion Colors Copper Green Oxidation State copper will start to react with the oxygen in the air to form copper oxide. For example, both [cu(nh 3 ). the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. . Copper Green Oxidation State.
From blog.thepipingmart.com
How to Remove Green Oxidation from Brass A Complete Guide Copper Green Oxidation State Copper (ii) complex ions, but there is also a substantial. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. the chemistry of copper is dominated by the +2 oxidation state, e.g. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of.. Copper Green Oxidation State.
From www.researchgate.net
The oxidation state of LFP Si6 compare with standard sampel Fe Kedge Copper Green Oxidation State This is what a reaction is called when atoms change their. The copper oxide will continue reacting to oxygen over time. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. copper. Copper Green Oxidation State.
From www.youtube.com
Determine the oxidation number oxidation state of each element in the Copper Green Oxidation State Copper (ii) complex ions, but there is also a substantial. the chemistry of copper is dominated by the +2 oxidation state, e.g. The copper oxide will continue reacting to oxygen over time. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. the two principal oxidation states of copper. Copper Green Oxidation State.
From www.doubtnut.com
If an element can exist in several oxidation states, it is convenient Copper Green Oxidation State The copper oxide will continue reacting to oxygen over time. Copper (ii) complex ions, but there is also a substantial. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. copper oxide. Copper Green Oxidation State.
From www.slideserve.com
PPT Experiment 3 PowerPoint Presentation, free download ID243127 Copper Green Oxidation State Copper (ii) complex ions, but there is also a substantial. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. the vinegar mixture causes a chemical reaction between the copper and. Copper Green Oxidation State.
From www.chegg.com
Solved This experiment is the Green Oxidation of (S)Borneol Copper Green Oxidation State the chemistry of copper is dominated by the +2 oxidation state, e.g. For example, both [cu(nh 3 ). copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. The copper oxide will continue reacting to oxygen over time. Copper (ii) complex ions, but there is also a substantial. the. Copper Green Oxidation State.
From www.youtube.com
Oxidation of Copper to Copper Oxide Class X Science Chapter1 Copper Green Oxidation State copper will start to react with the oxygen in the air to form copper oxide. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. the chemistry of copper. Copper Green Oxidation State.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic Copper Green Oxidation State the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. The copper oxide will continue reacting to oxygen over time. Copper (ii) complex ions, but there is also a substantial. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. forming. Copper Green Oxidation State.
From www.youtube.com
20 Oxidation states of Copper ch2 12th YouTube Copper Green Oxidation State forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. This is what a reaction is called when atoms change their. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. copper oxide is not green, but green verdigris, basic copper carbonate. Copper Green Oxidation State.
From www.youtube.com
How does the oxidation state of copper changes when Fehling solution is Copper Green Oxidation State the chemistry of copper is dominated by the +2 oxidation state, e.g. The copper oxide will continue reacting to oxygen over time. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. copper will start to react with the oxygen in the air to form copper oxide. For example,. Copper Green Oxidation State.
From docslib.org
Oxidation States of Carbon Oxidation and Reduction in Biology DocsLib Copper Green Oxidation State The copper oxide will continue reacting to oxygen over time. copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. For example, both [cu(nh 3 ). This is what a reaction is. Copper Green Oxidation State.
From www.researchgate.net
Speciation diagram showing the oxidation state and major species of Copper Green Oxidation State the chemistry of copper is dominated by the +2 oxidation state, e.g. copper will start to react with the oxygen in the air to form copper oxide. The copper oxide will continue reacting to oxygen over time. For example, both [cu(nh 3 ). the vinegar mixture causes a chemical reaction between the copper and the air known. Copper Green Oxidation State.
From www.researchgate.net
The different copper oxidation states, average copper oxidation state Copper Green Oxidation State forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. copper will start to react with the oxygen in the air to form copper oxide. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. Copper (ii) complex ions, but there is. Copper Green Oxidation State.
From www.thinkswap.com
Oxidation State Chemistry Form 5 SPM Thinkswap Copper Green Oxidation State the chemistry of copper is dominated by the +2 oxidation state, e.g. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. The copper oxide will continue reacting to oxygen over time. the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known.. Copper Green Oxidation State.
From www.pnas.org
Oxidation statespecific fluorescent copper sensors reveal oncogene Copper Green Oxidation State For example, both [cu(nh 3 ). the two principal oxidation states of copper are +1 and +2 although some +3 complexes are known. The copper oxide will continue reacting to oxygen over time. Copper (ii) complex ions, but there is also a substantial. the chemistry of copper is dominated by the +2 oxidation state, e.g. This is what. Copper Green Oxidation State.
From www.youtube.com
Neutral compounds In a neutral compound the sum of the oxidation state Copper Green Oxidation State copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. This is what a reaction is called when atoms change their. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. The copper oxide will continue reacting to oxygen over time.. Copper Green Oxidation State.
From pubs.acs.org
Protecting Copper Oxidation State via Intermediate Confinement for Copper Green Oxidation State copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. This is what. Copper Green Oxidation State.
From www.pnas.org
Oxidation statespecific fluorescent copper sensors reveal oncogene Copper Green Oxidation State copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. Copper (ii) complex ions, but there is also a substantial. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. This is what a reaction is called when atoms change their.. Copper Green Oxidation State.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Copper Green Oxidation State copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. the chemistry of copper is dominated by the +2 oxidation state, e.g. This is what a reaction is called when atoms change their. the two principal oxidation states of copper are +1 and +2 although some +3 complexes. Copper Green Oxidation State.
From blog.thepipingmart.com
Copper Oxidation States and Colors Copper Green Oxidation State the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. the chemistry of copper is dominated by the +2 oxidation state, e.g. forming copper(i) complexes (other than the one with water as a ligand) also stabalises the copper(i) oxidation state. For example, both [cu(nh 3 ). the two principal. Copper Green Oxidation State.
From quizlet.com
What is the oxidation number of \ce{Br} in \ce{Br3O8}? Quizlet Copper Green Oxidation State copper oxide is not green, but green verdigris, basic copper carbonate (or acetate) forms on copper exposed to air. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. For example, both [cu(nh 3 ). forming copper(i) complexes (other than the one with water as a ligand) also stabalises. Copper Green Oxidation State.
From pubs.acs.org
Oxidation State Dependent Conjugation Controls Electrocatalytic Copper Green Oxidation State This is what a reaction is called when atoms change their. copper has a beautiful reddish hue, but when exposed to the elements, the metal undergoes a series of. copper will start to react with the oxygen in the air to form copper oxide. forming copper(i) complexes (other than the one with water as a ligand) also. Copper Green Oxidation State.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Green Oxidation State the chemistry of copper is dominated by the +2 oxidation state, e.g. The copper oxide will continue reacting to oxygen over time. the vinegar mixture causes a chemical reaction between the copper and the air known as a redoxreaction. This is what a reaction is called when atoms change their. copper has a beautiful reddish hue, but. Copper Green Oxidation State.