Equivalence Point Titration Ammonia . Sorting out some confusing terms. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. choosing indicators for titrations. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration.
from www.chegg.com
Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The equivalence point of a titration. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. Sorting out some confusing terms. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. choosing indicators for titrations. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a.
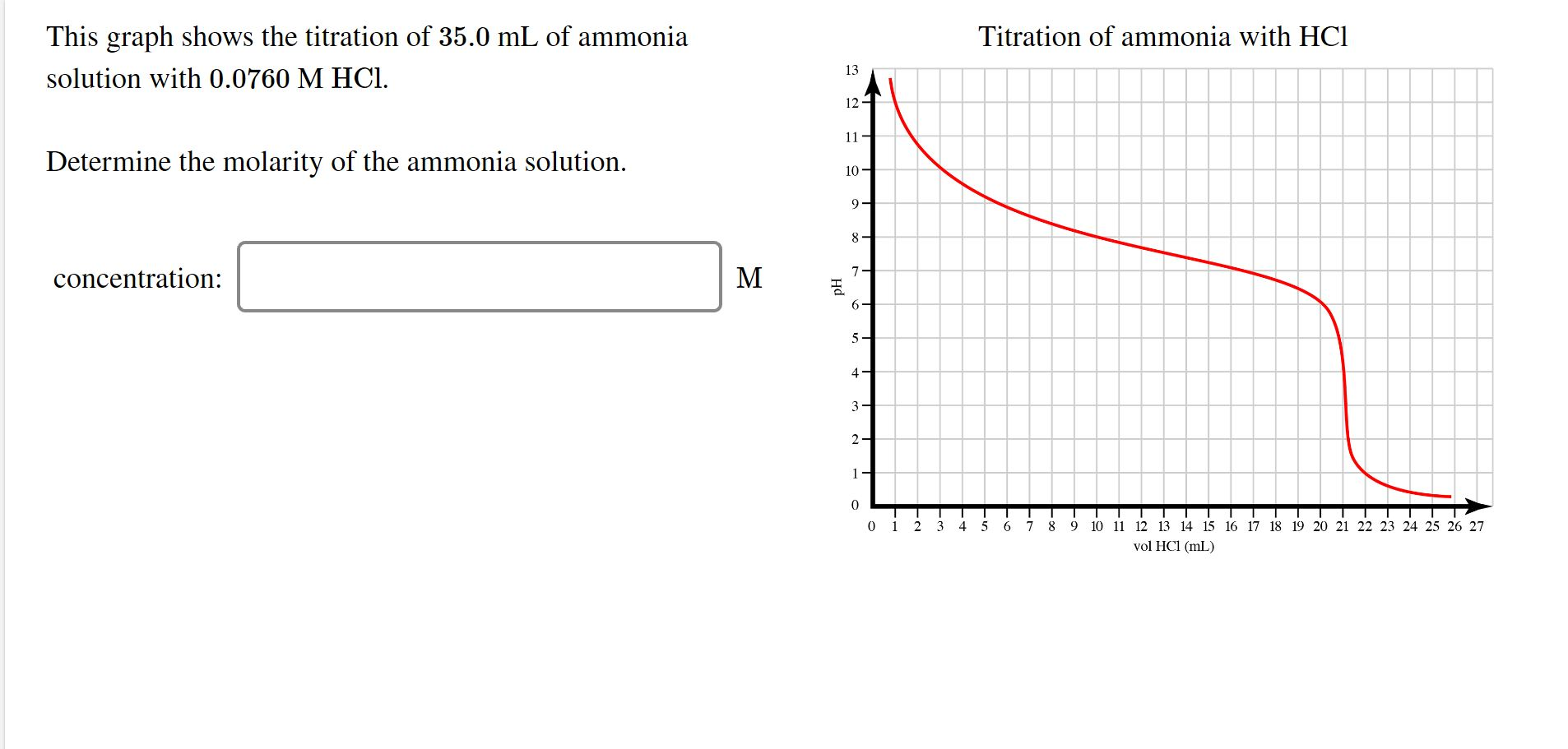
Solved Titration of ammonia with HCl This graph shows the
Equivalence Point Titration Ammonia to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. The equivalence point of a titration. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. choosing indicators for titrations. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution.
From chempedia.info
Ammonia titration curve Big Chemical Encyclopedia Equivalence Point Titration Ammonia Sorting out some confusing terms. The equivalence point of a titration. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the ammonia can be distilled into. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED Below is a titration curve for the titration of 100 mL 0.29 M Equivalence Point Titration Ammonia At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The equivalence point of a titration. Sorting out some confusing terms. this titrant addition involves a stoichiometric amount of base (the equivalence. Equivalence Point Titration Ammonia.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. at the equivalence point, equal numbers of moles of acid and base have been added and the. Equivalence Point Titration Ammonia.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Equivalence Point Titration Ammonia the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. Sorting out some confusing terms. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. choosing indicators for titrations. to evaluate. Equivalence Point Titration Ammonia.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Equivalence Point Titration Ammonia the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct. Equivalence Point Titration Ammonia.
From www.numerade.com
VIDEO solution Titration Curves Sketch the titration curve of an Equivalence Point Titration Ammonia to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. at the equivalence point, equal numbers of moles of acid and base have been added and. Equivalence Point Titration Ammonia.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point Equivalence Point Titration Ammonia choosing indicators for titrations. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. The equivalence point of a titration. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the ammonia can be. Equivalence Point Titration Ammonia.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Equivalence Point Titration Ammonia to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED What reaction can occur between two of the species present at Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The equivalence point of a titration. Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the ammonia can be distilled into an excess of. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED Part Titration of Ammonia Base (Attach Graph) mL Vinflection mL Equivalence Point Titration Ammonia The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Remember that the equivalence point of a titration is where you have mixed the two substances. Equivalence Point Titration Ammonia.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point Titration Ammonia At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are. Equivalence Point Titration Ammonia.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Equivalence Point Titration Ammonia this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. at the equivalence point, equal numbers of moles of acid and base have been added and. Equivalence Point Titration Ammonia.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Equivalence Point Titration Ammonia The equivalence point of a titration. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. choosing indicators for titrations. the ammonia can be distilled. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED In the titration of 0.100 M ammonia (NH3) with 0.100 M HCI, the Equivalence Point Titration Ammonia The equivalence point of a titration. Sorting out some confusing terms. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. choosing indicators for titrations. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect. Equivalence Point Titration Ammonia.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Equivalence Point Titration Ammonia at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. The equivalence point of a titration. Remember that the equivalence point of. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED What reaction can occur between two of the species present at Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. to evaluate the relationship between a titration’s equivalence point and its end point we need to. Equivalence Point Titration Ammonia.
From dxomjwenv.blob.core.windows.net
Titration Half Equivalence Point at Karen Winkleman blog Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. choosing indicators for titrations. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. Sorting out some confusing terms. The equivalence point of a titration. At the. Equivalence Point Titration Ammonia.
From www.researchgate.net
Titration of an ammonia PT. The blue line shows the pH of the KCl Equivalence Point Titration Ammonia to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Sorting out some confusing terms. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. The equivalence point of a titration. choosing indicators for titrations.. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED A student is doing a titration of a 50.0 mL ammonia (NH3 Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. At the equivalence point in a neutralization, the moles of acid are equal to the moles of. Equivalence Point Titration Ammonia.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point Titration Ammonia the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. The equivalence point of a titration. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. to evaluate the relationship between a. Equivalence Point Titration Ammonia.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVEDC Titration Curves (10 pts ) Why is the equivalence point less Equivalence Point Titration Ammonia The equivalence point of a titration. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. this titrant addition involves a stoichiometric. Equivalence Point Titration Ammonia.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Equivalence Point Titration Ammonia at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. choosing indicators for titrations. Remember that the equivalence point of a titration. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED A student is doing a titration of a 50.0 mL ammonia (NH3 Equivalence Point Titration Ammonia The equivalence point of a titration. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. at the equivalence point, equal numbers of moles of acid and base have been. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED Acid HCl and hydrochloric = ammonia, NH3. Given the titration Equivalence Point Titration Ammonia at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. choosing indicators for titrations. Remember that the equivalence point of a. Equivalence Point Titration Ammonia.
From exoxoimsp.blob.core.windows.net
Point Titration Definition at Gerald Lathrop blog Equivalence Point Titration Ammonia At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. at the equivalence point, equal numbers of moles of acid and base have been added and the ph. Equivalence Point Titration Ammonia.
From www.chegg.com
Solved Titration of ammonia with HCl This graph shows the Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction. Equivalence Point Titration Ammonia.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. Sorting out some confusing terms. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. At the equivalence point in a neutralization, the moles of acid are equal. Equivalence Point Titration Ammonia.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point Titration Ammonia Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. Sorting out some confusing terms. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. At the equivalence point in a neutralization, the moles of acid are equal. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVED Steps plz 2. Given the titration curve for a titration between Equivalence Point Titration Ammonia to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. choosing indicators for titrations. Sorting out some confusing terms. at the equivalence point, equal. Equivalence Point Titration Ammonia.
From www.researchgate.net
Top ammonia titration curve (pH¼f(V)) in boric acid (LEFT) and Gran Equivalence Point Titration Ammonia Sorting out some confusing terms. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. this titrant addition involves a stoichiometric amount of base (the equivalence point), and. Equivalence Point Titration Ammonia.
From www.chegg.com
Solved 3 What is the pH at the equivalence point in the Equivalence Point Titration Ammonia At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species. Equivalence Point Titration Ammonia.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Equivalence Point Titration Ammonia to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The equivalence point of a titration. choosing indicators for titrations. the ammonia can be distilled into an excess of. Equivalence Point Titration Ammonia.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog Equivalence Point Titration Ammonia the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. The equivalence point of a titration. at the equivalence point, equal numbers of moles of acid and base have been added and the ph will reflect which species are. to evaluate the relationship between a. Equivalence Point Titration Ammonia.
From www.numerade.com
SOLVEDYou require 36.78 mL of 0.0105 M HCl to reach the equivalence Equivalence Point Titration Ammonia The equivalence point of a titration. the ammonia can be distilled into an excess of standard strong acid (hcl or h 2 so 4), and the excess determined. Sorting out some confusing terms. this titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution. At the. Equivalence Point Titration Ammonia.